News
20242023202220212020
David Gill to Lead Cognitive and Behavioral Neurology
Monday, April 1, 2024

David Gill, MD, has been appointed chief of the Division of Cognitive and Behavioral Neurology at the University of Rochester Medical Center (URMC). A highly respected local clinician and academic, Gill returns to the institution where he completed his residency and fellowship training and assumes his new position effective April 1, 2024.
‘Working in collaboration with our colleagues in Psychiatry, Dave has an exciting vision for the future of dementia care in our community,” said Robert Holloway, MD, MPA, chair of URMC Department of Neurology. “He will help grow the Memory Care Program, improve access to cutting-edge treatments for patients and families in a socially responsible manner, and expand training opportunities to increase the numbers of providers that care for patients with cognitive disorders, specifically in the Rochester area.”
Gill will work with the teams in the Alzheimer’s Disease Care, Research and Education (AD-CARE) Program, the Del Monte Neurosciences Institute, and the University of Rochester Aging Institute to support and help grow basic science and clinical research programs in the field of cognitive disorders. Gill will also build upon his existing relationships in the community to advance education for patients and caregivers through partnerships with Lifespan, the Alzheimer’s Association, and other community groups.
Read More: David Gill to Lead Cognitive and Behavioral NeurologyJamie Capal Tapped as New Head of Child Neurology
Friday, February 9, 2024
 Jamie Capal, MD, has been selected the next chief of the Division of Child Neurology at the University of Rochester Medical Center (URMC) and Golisano Children’s Hospital. Capal—who specializes in intellectual and developmental disabilities—joins a program that has, though more than 50 years of clinical innovation and research, transformed care for children with neurological disorders.
Jamie Capal, MD, has been selected the next chief of the Division of Child Neurology at the University of Rochester Medical Center (URMC) and Golisano Children’s Hospital. Capal—who specializes in intellectual and developmental disabilities—joins a program that has, though more than 50 years of clinical innovation and research, transformed care for children with neurological disorders.
“Jamie has an exciting vision to lead our pre-eminent program in Child Neurology to even greater heights,” said Bob Holloway, MD, MPH, chair of the Department of Neurology. “She will use her prior experiences to mentor faculty, providers, trainees, and staff to build and support successful teams around the many priority programs within the division and across the Medical Center. She will also play an instrumental role in intellectual and developmental disabilities research and the growth of our rare disease and gene therapy clinical trials operations.”
Capal will hold the Frederick A. Horner, MD, Endowed Distinguished Professorship in Pediatric Neurology and will also have an appointment in the Department of Pediatrics. She will also serve as co-director of the Human Clinical Phenotyping and Recruitment Core of the University of Rochester Intellectual Developmental Disability Research Center (UR-IDDRC). She will start her new position on July 1, 2024
“Intellectual and development disabilities are one of the cornerstones of the University’s neuroscience research enterprise, and we are only one of a handful of institutions that hold the three major federally-funded centers of excellence in research, training, care, and community partnership in this field,” said John Foxe, PhD, director of the University of Rochester Del Monte Institute for Neuroscience and co-director of the UR-IDDRC. “These are some of the most complex and challenging diseases in all of medicine, and Dr. Capal’s clinical research experience will enable us to continue to build bridges between the research and clinical programs, and ultimately improve care.”
Read More: Jamie Capal Tapped as New Head of Child NeurologyCalcium Channel Blockers Key to Reversing Myotonic Dystrophy Muscle Weakness, Study Finds
Tuesday, January 2, 2024
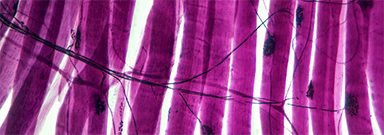 New research has identified the specific biological mechanism behind the muscle dysfunction found in myotonic dystrophy type 1 (DM1) and further shows that calcium channel blockers can reverse these symptoms in animal models of the disease. The researchers believe this class of drugs, widely used to treat a number of cardiovascular diseases, hold promise as a future treatment for DM1.
New research has identified the specific biological mechanism behind the muscle dysfunction found in myotonic dystrophy type 1 (DM1) and further shows that calcium channel blockers can reverse these symptoms in animal models of the disease. The researchers believe this class of drugs, widely used to treat a number of cardiovascular diseases, hold promise as a future treatment for DM1.
“The main finding of our study is that combined calcium and chloride channelopathy is highly deleterious and plays a central role in the function impairment of muscle found in the disease,” said John Lueck, PhD, an assistant professor at the University of Rochester Medical Center (URMC) in the Departments of Pharmacology and Physiology, and Neurology. “Our research also suggests that muscle impairment in DM1 is potentially mitigated by common clinically available calcium channel blockers and that calcium channel modulation is a potential therapeutic strategy.” Lueck is lead author of the study, which appears in the Journal of Clinical Investigation.
Toxic RNA disrupts muscle function
Myotonic dystrophy is one of the most common forms of muscular dystrophy. People with the disease have muscle weakness and prolonged muscle tensing (myotonia), making it difficult to relax muscles after use. The disease also affects the eyes, heart, and brain, leading eventually to difficulty walking, swallowing, and breathing.
More than 20 years ago, URMC neurologist Charles Thornton, MD, and others uncovered how a genetic flaw–a trinucleotide repeat expansion that results in thousands of repetitions of code on a segment of chromosome 19–gives rise to DM1. This repeat expansion, which grows longer over time, results in the creation of abnormal RNA which accumulates in the nucleus of cells and interferes with the normal processing of many other RNAs. Thornton is a co-author of the current study and the research was a collaboration between the Lueck and Thornton labs.
Read More: Calcium Channel Blockers Key to Reversing Myotonic Dystrophy Muscle Weakness, Study FindsResident Interviews
Monday, January 1, 2024
The Department of Neurology would like to welcome residency applicants, interviewing on Monday, January 8th
Child Neurology Applicants
Naomi Cohen – The University of Texas Southwestern Medical School
Micailya Hall – Eastern Virginia Medical School
Melissa Huberman – University of Miami
Lucy Laura Olivieri – University of New England
Adryanna Tucker – Edward Via College of Osteopathic Medicine