Projects
Developing Targeted Drug Delivery Systems to Treat Leukemia
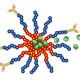 Acute myeloid leukemia (AML) recurrences are attributed to leukemia stem cells (LSCs) and a harsh marrow microenvironment that supports AML cell survival. LSCs in particular survive conventional chemotherapy and radiation treatments leading to relapse in patients. Drugs such as parthenolide (PTL) and micheliolide have has shown remarkable efficacy in inducing selective apoptosis in LSCs. However, these compounds’ low water solubility prevents them from reaching therapeutically effective levels in the blood stream. To circumvent this problem, we are developing a novel micelle delivery system to solubilize and target compounds.
Acute myeloid leukemia (AML) recurrences are attributed to leukemia stem cells (LSCs) and a harsh marrow microenvironment that supports AML cell survival. LSCs in particular survive conventional chemotherapy and radiation treatments leading to relapse in patients. Drugs such as parthenolide (PTL) and micheliolide have has shown remarkable efficacy in inducing selective apoptosis in LSCs. However, these compounds’ low water solubility prevents them from reaching therapeutically effective levels in the blood stream. To circumvent this problem, we are developing a novel micelle delivery system to solubilize and target compounds.
Learn more about Developing Targeted Drug Delivery Systems to Treat Leukemia
Developing Tissue Mimetics of the Outer Retinal Blood Barrier
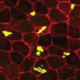 Age-related macular degeneration (AMD) is the leading cause of blindness in adults >50 years old in the US and its incidence worldwide is expected to double by 2020. The widely used rodent models of macular degeneration have vastly increased our knowledge of plausible disease mechanisms. However, the anatomical and physiological differences in human versus rodent retina have stymied both fundamental study of AMD progression and development of new therapies to treat AMD. To overcome this major hurdle, the goal of this work is to develop relevant tissue mimetics using hydrogel materials and cells from affected patients using human induced pluripotent stem cell (hiPSC) technology. This human tissue mimetic is of the outer retinal blood barrier-vasculature, or the retinal pigment epithelium-choriocapillaris (RPE-CC) complex. This mimetic will provide a robust platform to study the role of individual cell types (RPE versus CC) in the pathophysiology of several eye diseases including AMD.
Age-related macular degeneration (AMD) is the leading cause of blindness in adults >50 years old in the US and its incidence worldwide is expected to double by 2020. The widely used rodent models of macular degeneration have vastly increased our knowledge of plausible disease mechanisms. However, the anatomical and physiological differences in human versus rodent retina have stymied both fundamental study of AMD progression and development of new therapies to treat AMD. To overcome this major hurdle, the goal of this work is to develop relevant tissue mimetics using hydrogel materials and cells from affected patients using human induced pluripotent stem cell (hiPSC) technology. This human tissue mimetic is of the outer retinal blood barrier-vasculature, or the retinal pigment epithelium-choriocapillaris (RPE-CC) complex. This mimetic will provide a robust platform to study the role of individual cell types (RPE versus CC) in the pathophysiology of several eye diseases including AMD.
Learn more about Developing Tissue Mimetics of the Outer Retinal Blood Barrier
Hydrogels for Local Delivery of Therapeutics
 Hydrogels can be exploited to encapsulate and deliver cells and biomolecules therapeutically, as delivery characteristics can be intimately controlled through alterations in hydrogel biophysical and biochemical structure. We are interested in using hydrogels to in situ polymerize delivery systems for cells and/or therapeutics that act locally to aid ischemic tissue regeneration, wound healing, or control inflammation.
Hydrogels can be exploited to encapsulate and deliver cells and biomolecules therapeutically, as delivery characteristics can be intimately controlled through alterations in hydrogel biophysical and biochemical structure. We are interested in using hydrogels to in situ polymerize delivery systems for cells and/or therapeutics that act locally to aid ischemic tissue regeneration, wound healing, or control inflammation.
Learn more about Hydrogels for Local Delivery of Therapeutics
pH-Responsive Nanoparticle Drug Delivery to Inhibit Oral Biofilm Formation
 Tooth decay, often referred to as cavities and known clinically as dental caries, is caused by an oral biofilm (i.e., dental plaque) and affects nearly all adults worldwide, costing billions of dollars annually. Current treatment options using topically applied drugs offer little to no protection due to the poor solubility or salivary clearance of drugs as well as a protective barrier created by the biofilm. Therefore, our lab developed polymer nanoparticles that can load poorly soluble drugs, penetrate dental plaque, and release those drugs due to changes in local pH conditions precisely where cavities develop: the acid-covered tooth surface.
Tooth decay, often referred to as cavities and known clinically as dental caries, is caused by an oral biofilm (i.e., dental plaque) and affects nearly all adults worldwide, costing billions of dollars annually. Current treatment options using topically applied drugs offer little to no protection due to the poor solubility or salivary clearance of drugs as well as a protective barrier created by the biofilm. Therefore, our lab developed polymer nanoparticles that can load poorly soluble drugs, penetrate dental plaque, and release those drugs due to changes in local pH conditions precisely where cavities develop: the acid-covered tooth surface.
Learn more about pH-Responsive Nanoparticle Drug Delivery to Inhibit Oral Biofilm Formation
Radioprotection and Regeneration of Salivary Glands
 For more than 50,000 patients a year in the U.S. diagnosed with head and neck cancers, severe loss of salivary gland function is an unavoidable outcome of radiation therapy used in treatment. A direct way to prevent xerostomia is to protect the salivary gland cells from radiation damage. Thus, we developed a radioprotective strategy using siRNA-nanoparticle complexes to block genes associated with radiation damage. This strategy results in a remarkable preservation of the histology and function in the treated salivary glands and current work is focused on the long-term safety and efficacy of this rescue.
For more than 50,000 patients a year in the U.S. diagnosed with head and neck cancers, severe loss of salivary gland function is an unavoidable outcome of radiation therapy used in treatment. A direct way to prevent xerostomia is to protect the salivary gland cells from radiation damage. Thus, we developed a radioprotective strategy using siRNA-nanoparticle complexes to block genes associated with radiation damage. This strategy results in a remarkable preservation of the histology and function in the treated salivary glands and current work is focused on the long-term safety and efficacy of this rescue.
Learn more about Radioprotection and Regeneration of Salivary Glands
Targeted Drug Delivery Systems for Musculoskeletal Applications
 My laboratory has unique expertise in polymeric materials development and has exploited this approach to overcome challenges associated in musculoskeletal tissue-based delivery. These challenges include poor tissue-specific biodistribution and resulting off-target effects. We employ a controlled/living polymerization strategy known as RAFT (reversible-addition fragmentation chain transfer) to synthesize polymers with well-controlled molecular weight, polydispersities, flexible polymer chain end functionalities, and a multitude of architectures, all attributes enabling reproducible, effective, and targeted therapeutic delivery.
My laboratory has unique expertise in polymeric materials development and has exploited this approach to overcome challenges associated in musculoskeletal tissue-based delivery. These challenges include poor tissue-specific biodistribution and resulting off-target effects. We employ a controlled/living polymerization strategy known as RAFT (reversible-addition fragmentation chain transfer) to synthesize polymers with well-controlled molecular weight, polydispersities, flexible polymer chain end functionalities, and a multitude of architectures, all attributes enabling reproducible, effective, and targeted therapeutic delivery.
Learn more about Targeted Drug Delivery Systems for Musculoskeletal Applications
Tissue Engineered Periosteum Approaches to Heal Bone Allograft Transplants
 Although most orthopaedic fractures heal, the clinical management of critical (>3mm) segmental defects continues to present major challenges for both amputation and limb salvage approaches. The periosteum plays an essential role in the healing process of both fractures and autografts. Periosteal stem cells have been shown necessary for the induction of robust endochondral and intramembraneous bone formation, essential for effective healing and neovascularization of autografts.
Although most orthopaedic fractures heal, the clinical management of critical (>3mm) segmental defects continues to present major challenges for both amputation and limb salvage approaches. The periosteum plays an essential role in the healing process of both fractures and autografts. Periosteal stem cells have been shown necessary for the induction of robust endochondral and intramembraneous bone formation, essential for effective healing and neovascularization of autografts.
Learn more about Tissue Engineered Periosteum Approaches to Heal Bone Allograft Transplants