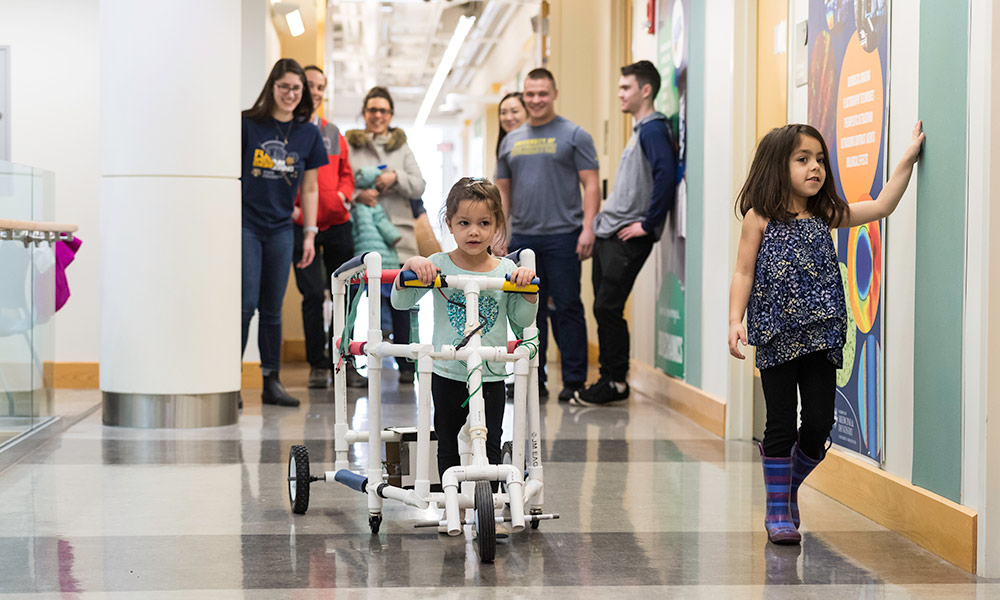
Louisa Buckley, accompanied by her sister Rosemary — daughters of assistant professor Mark Buckley — helps a team of biomedical engineering seniors test out a prototype of their early childhood mobility device. (University of Rochester photo / J. Adam Fenster)
Making their mark: This is one in a series of profiles celebrating members of Rochester’s graduating class of 2018.
For young children with Down syndrome, cerebral palsy, and other developmental disabilities, learning to walk can be a long-term process. And in the meantime, the children find it hard to keep up with their peers, which increases their social isolation.
A team of biomedical engineering majors, working with Leah Talbot, a Rochester area physical therapist, believes it can address both issues with an inexpensive, “hybrid” walker that will be portable enough to accompany the children wherever they go.
“This is right up our alley,” says Joe Cappotelli ’18, whose senior design project teammates are Hyun Choi ’18, Devon Foggio ’18, and Daniel Myers ’18. “We’re all in the biomechanics track of biomedical engineering. And it’s a fun project, to be able to think about ways we could help these children in the future.”
When the team surveyed what is currently available, they found “go-baby-go” cars — ordinary toy cars turned into personalized vehicles for young children with disabilities. These enable the 3- to 5-year-olds to keep up with their peers, but aren’t that helpful from a therapeutic standpoint, because they don’t require the children to actually propel themselves.
Walkers used in clinical settings, on the other hand, are often bulky and expensive: great for therapy, but not for keeping up with more mobile playmates, or for taking home.
“We’ve created sort of a hybrid of the two,” says Myers. “This allows them to move around but also practice walking at the same time.” And, Foggio adds, at a more reasonable cost compared to the therapeutic devices used in clinical settings, which range from $700 to $1,200. The walker the students have designed would only cost $150 to 200, they estimate. “We wanted to find an in between,” Foggio says.
The walker consists of a frame of relatively light-weight plastic tubing, an adjustable harness in the center to support the child, steering column, and a motor/gear box/rear axle assembly to propel it.