URMC / Labs / Frelinger Lab / Projects / Tumor Immunotherapy
Tumor Immunotherapy
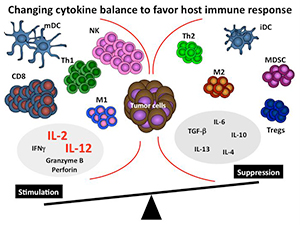
Figure 1. Changing cytokine balance to favor host
immune response.
We have a long-standing interest in the immune response to tumors. Like many immunologists, we recognized the tremendous potential of cytokines and showed experimentally that they played a key role in anti-tumor responses. We have focused on the effects of cytokines within the tumor microenvironment.
Below is a simplified schematic of the tumor microenvironment. Note that the tumor microenvironment contains many different cells and cytokines, some with opposing functions. Our overall hypothesis is that certain key cytokines are critical for establishing and maintaining the overall nature of the immune response to tumors. By altering the cytokine milieu at the tumor site using cytokines such as IL-2 or IL-12 we propose that we will be able to convert this immunosuppressive microenvironment to one that promotes and enhances an immune response (see figure 1).
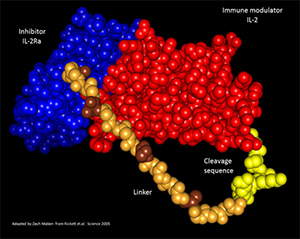
Figure 2. Composite crystal structure of hIL-2/IL-2Rα
fusion protein. Shown is the crystal structure of
human IL-2 (red) bound to the IL-2Rα chain (blue)
separated by a repeating linker (brown/tan) as well
as a protease cleavage sequence (yellow) adapted
from the actual crystal structure published by Garcia
Group at Stanford. This model was generated by
Zach Maben in my laboratory.
The precise properties that make cytokines attractive for therapy, namely their potent and pleiotropic effects, make their systemic use problematic.
We are developing novel ways to overcome this limitation. We have been developing novel fusion proteins that can be activated preferentially at the tumor site by proteases that are over expressed.
We have termed this PACman (for protease activated cytokine) approach. If successful, one should be able to delivers cytokines systemically but have them become functionally active preferentially at the tumor site. We have constructed an IL-2 fusion protein that shows dramatic increase of IL-2 functional activity after proteases cleavage. A model of this fusion protein is shown in figure 2.
These fusion proteins are now being tested in several tumor models including the omental tumor model that we have developed in collaboration with Dr. Edith Lord (see figure 3).
The omentum model allows one to examine tumor growth at a common site of metastases. We have shown that fusion protein delivery can reduce the tumor growth at this site and this model is also amenable to examining human ovarian tumors in xenografts (also called the OTX model).
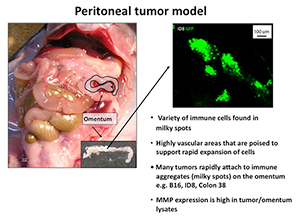
Figure 3.
These activatable fusion proteins are novel research tools and will allow us to determine how cytokine perturbations affect the host response to tumors. Importantly, these cytokine fusion proteins may also be used as therapeutics for cancer therapy that harnesses the immense potential of cytokines.
We believe this strategy may ultimately be used for cancer treatment for a variety of cancers including melanoma, breast, ovarian, and colon.
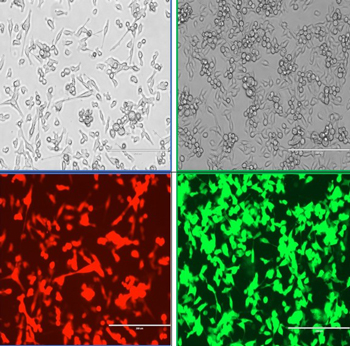
Figure 4: Images of tumors that have been transfected to express either a red (Kate2) or green (GFP) fluorescent protein. Shown are Line 1 lung cells in bright field or fluorescent images. Cells were transfected by Shannon Ferry and Nolan Kearns.
The video below highlights the fusion protein concept.
« back to all projects