Breakthrough in Understanding New Class of Antibiotics
Thanks to researchers at the University of Rochester School of Medicine and Dentistry, we are now a step closer to understanding how to combat antibiotic-resistant superbugs.
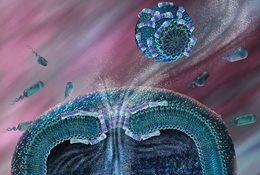
Dejun Lin, doctoral candidate in the Department of Biochemistry and Biophysics and Alan Grossfield, Ph.D., associate professor of Biochemistry and Biophysics at the University of Rochester School of Medicine and Dentistry have found out how a new class of antibiotic drugs kill bacteria without harming their host. AMLPs (antimicrobial lipopeptides) poke holes in bacterial membranes leading to their demise, but tend to leave mammalian cells unscathed.
The finding is welcome news: According to the Centers for Disease Control and Prevention, 23,000 people die from infection with antimicrobial-resistant bacteria each year in the United States.
It was unknown how AMLPs spared the host until Lin and Grossfield published their recent study in Biophysical Journal. The pair employed computer modeling to understand how individual molecules of AMLPs interact with one another as well as with cellular membranes of both bacteria and mammalian cells. The key, they found, is that AMLPs form highly structured balls, called micelles, before they interact with cellular membranes.
According to their calculations, individual AMLPs are highly likely to bind to any membrane - whether mammalian or bacterial. “Any time one of these guys encounters ANY membrane, it’s going to stick,” says Grossfield. “That seems wrong because in the body there are a lot more mammalian cells than there are bacterial cells. How would it ever get to a bacterial cell if it sticks to the first thing that it hits?”
When AMLPs gather into micelles, on the other hand, they only attack bacterial membranes. “In the case of bacteria, the membrane is negatively charged and the micelle is positively charged, so the micelle sticks on the surface and eventually opens up, creating a hole in the membrane. For the mammalian membrane, the micelle doesn’t want to stick. In fact, it’s repelled,” says Grossfield.
Because AMLPs target cellular membranes, bacteria are not likely to be able to evolve to become resistant to them. Current antibiotics tend to target and damage single molecules that are essential for bacteria to thrive. Over time, the bacteria mutate or change the molecule under fire so that the antibiotic no longer recognizes it. This may only require a single mutation in the bacteria, but has rendered antimicrobial drugs ineffective against certain bugs. In contrast, in order to resist AMLPs, bacteria would have to mutate many molecules and drastically change the make up of its membrane, which would likely be a suicide mission.
Lin and Grossfield’s findings support previous studies that have captured images of AMLPs in micelle form and have shown that AMLPs can effectively clear bacterial infections in plants and fungal infections mice. However, the major contribution of their study is that it offers another “knob to tweak” in the process of developing AMLP compounds into safe and effective drugs.
AMLPs are designed to mimic natural antimicrobial peptides (AMPs) produced by most organisms. Despite widespread interest, AMPs make lousy antibiotics, because they are rapidly degraded in the blood stream and are likely to cause an immune reaction. However, AMLPs administered as pre-formed micelles, might avoid these problems.
Now that Lin and Grossfield have a handle on why AMLPs prefer bacteria, they plan to investigate if these drugs have a similar preference for fungi.
To download the full study, click here
###
The University of Rochester Medical Center is home to approximately 3,000 individuals who conduct research on everything from cancer and heart disease to Parkinson’s, pandemic influenza, and autism. Spread across many centers, institutes, and labs, our scientists have developed therapies that have improved human health locally, in the region, and across the globe. To learn more, visit http://www.urmc.rochester.edu/research.

