News
20242023202220212020
UR Center for RNA Biology Members and Alumni Attend RNA 2023 in Singapore
Friday, June 9, 2023

Pictured above, from left to right: Jessica Perciaccante (Genetics Grad Student, Anderson Lab); Douglas Anderson, PhD (Assistant Professor, Medicine, Aab CVRI); Yi-Tao Yu, PhD (Professor of Biochemistry & Biophysics); Xin Li, PhD (former UR Biochemistry & Biophysics Faculty, currently Professor at Zhejiang University); Sean Lindley (Biology Grad Student, Anderson Lab); Eric Phizicky, PhD (Professor of Biochemistry & Biophysics); Lynne Maquat, PhD (Professor of Biochemistry & Biophysics; Director of the UR CRB ); Eric J. Wagner, PhD (Professor of Biochemistry & Biophysics; Associate Director of the UR CRB); Weifeng Gu, PhD (UR Alumn – PhD 2005, currently Assistant Professor, UCR); Tatsuaki Kurosaki, PhD (Research Assistant Professor, Maquat Lab); Sara Ali (Biophysics Grad Student, Mathews Lab), Yi Pan (Biochemistry Grad Student, Yu Lab)
A large number of UR Center for RNA Biology members and alumni attended the 28th Annual RNA Society Meeting (‘RNA 2023’) held at the Suntec Convention Centre from May 30th-June 4th, 2023. In addition to those pictured, Douglas Turner, PhD, Professor Emeritus of the Department of Biochemistry and Member Emeritus of the Center for RNA Biology, attended and received the 2023 The RNA Society/Cold Spring Harbor Laboratory Press Distinguished Research Mentor Award, which recognizes outstanding mentorship by RNA Society members and highlights the importance of fostering the academic and professional development of trainees in RNA research.
Read More: UR Center for RNA Biology Members and Alumni Attend RNA 2023 in SingaporeA Search Engine for mRNA: Algorithm Identifies Optimal Sequences to Improve COVID Vaccines
Friday, May 19, 2023
Messenger RNA vaccines proved their worth in the COVID pandemic, and new software stands to make the already transformative technology even more powerful.
Scientists developed an algorithm to identify the most stable, efficient mRNA sequences for vaccines. Tests show that the algorithm-derived mRNAs resist deterioration longer, produce more COVID spike protein, and dramatically increase antibody levels in mice compared to currently used mRNA vaccines. The results were reported in the journal Nature.
Study authors believe their tool will be valuable to companies that make mRNA vaccines and to research teams developing mRNA-based therapies for genetic disorders, cancer and a plethora of other diseases that can be treated by using mRNA to express a needed protein.
Searching for the Strongest mRNA
The COVID shots given throughout the pandemic have many advantages—scalable production, safety, efficacy—but suffer from some big drawbacks, including the need for ultra-cold storage and the resultant distribution challenges, and waning immunity. These limitations are due to the fact that mRNAs are inherently unstable and prone to degradation (they are constantly being “eaten” by enzymes present in cells).
The “secret sauce” for creating stronger mRNA sequences requires the right balance of two factors: structure and genetic code. Past research shows that mRNAs with a tight, rigid structure, as opposed to a floppy, unconfined structure, degrade more slowly (structure consolidates mRNAs and provides protection from hungry enzymes). Consequently, they stay in cells for a longer period and have more time to make the desired protein.
The mRNA used in COVID vaccines directs our bodies to make the COVID spike protein. The number of mRNA sequences that encode the spike protein is enormous—larger than the number of atoms in the universe. But, some of these genetic instructions are more efficient than others: one set may allow cells to churn out protein more quickly, while a different set might have redundancies that lead to sluggish protein production.
So, how do you find the right combination of structure and code? RNA expert David Mathews, MD, PhD and computer scientist Liang Huang, PhD, collaborated to create an algorithm that assesses both factors. Like a Google search for mRNA sequences, their algorithm spits out the top result for a specific protein amongst the almost infinite number of possibilities.
“Our tool is designed to identify the best sequence out of a huge space that you could never explore experimentally,” said Mathews, co-corresponding author of the Nature study and the Lynne E. Maquat professor of Biochemistry and Biophysics at the University of Rochester Medical Center. “Prior approaches did a poor job of searching this space. We hope this breakthrough will help companies to develop or improve their mRNA therapies.”
Read More: A Search Engine for mRNA: Algorithm Identifies Optimal Sequences to Improve COVID VaccinesDavid Mathews Publishes Study in Nature on Algorithm to Optimize mRNA Vaccines
Thursday, May 4, 2023
The professor of Biochemistry and Biophysics partnered with an Oregon State University computer scientist to develop the algorithm to help research teams find the most stable, efficient mRNA sequences for vaccines and other therapies, such as monoclonal antibodies and anti-cancer drugs. Tested in COVID-19 mRNA vaccines, the algorithm substantially increased protein expression and antibody levels compared to currently used mRNA sequences.
Read More: David Mathews Publishes Study in Nature on Algorithm to Optimize mRNA VaccinesPulling the Plug on Viral Infections: CRISPR isn’t Just About Cutting
Thursday, April 27, 2023
Study shows how a Cas protein partners with a unique membrane protein to stop viral infection
CRISPR claimed scientific fame for its ability to quickly and accurately edit genes. But, at the core, CRISPR systems are immune systems that help bacteria protect themselves from viruses by targeting and destroying viral DNA and RNA. A new study published in Science reveals a previously unrecognized player in one such system – a membrane protein that enhances anti-viral defense – simultaneously broadening our understanding of and raising more questions related to the complexities of CRISPR.
Uncovering New Clues about CRISPR
CRISPR systems consist of two major components – a guide RNA that targets a specific viral DNA or RNA sequence and a Cas enzyme that cuts the targeted DNA or RNA, preventing a virus from replicating and spreading. A team at the University of Rochester Center for RNA Biology found that a specific Cas protein (Cas13b) not only cuts viral RNA, but communicates with another protein (Csx28) to augment its anti-viral defense.
In partnership with scientists at Cornell, the team discovered that the Csx28 protein forms a pore-like structure (i.e. it has a big hole in it). When they infected E. coli with a phage (virus that attacks bacteria) and deployed the CRISPR-Cas13 system to target and halt infection, they found that Cas13 signals to Csx28 to affect membrane permeability. Once this happens, Csx28 wreaks havoc in the infected cell, discombobulating membrane potential, crushing metabolism and hindering energy production. A virus can’t replicate under such unhospitable circumstances, leading to the team’s conclusion that Csx28 enhances CRISPR-Cas13b’s phage defense.
“This finding upends the idea that CRISPR systems mount their defense only by degrading RNA and DNA in cells and really broadens our view of how CRISPR systems may be working,” said corresponding author Mitchell O’Connell, PhD, assistant professor of Biochemistry and Biophysics at the University of Rochester Medical Center (URMC) and a member of the UR Center for RNA Biology. “When we think about CRISPR, we see Cas proteins such as Cas9 or Cas13 as the big hammer doing all the damage, but that might not be the case; we found that Cas13 and Csx28 are working together to effectively extinguish a virus.”
“When you read this paper you think to yourself…‘what?’ This is such a weird mechanism and not the way I would have predicted that bacteria would work,” added John Lueck, PhD, assistant professor of Pharmacology and Physiology at URMC. “It is really impressive that the team identified this pore-like protein that doesn’t resemble anything else we’ve seen before, and now that we know that this mechanism exists people will start to look for it in other systems. This is exciting because in science, when you scratch the surface, you often find that there is an entirely new world behind it.”
Read More: Pulling the Plug on Viral Infections: CRISPR isn’t Just About Cutting2023 RNA Institute Symposium: UR Talks and Awards
Tuesday, April 4, 2023
 The 2023 RNA Institute Symposium, a collaborative event hosted by The RNA Institute at the University at Albany (UAlbany) and the University of Rochester (UR) Center for RNA Biology, was held March 16-17, 2023, at UAlbany. This two-day event was co-organized by Lynne Maquat, PhD, Dave Matthews, MD, PhD, Eric Wagner, PhD from the UR Center for RNA Biology, and J. Andrew Berglund, PhD and Marlene Belfort, PhD from The RNA Institute at UAlbany.
The 2023 RNA Institute Symposium, a collaborative event hosted by The RNA Institute at the University at Albany (UAlbany) and the University of Rochester (UR) Center for RNA Biology, was held March 16-17, 2023, at UAlbany. This two-day event was co-organized by Lynne Maquat, PhD, Dave Matthews, MD, PhD, Eric Wagner, PhD from the UR Center for RNA Biology, and J. Andrew Berglund, PhD and Marlene Belfort, PhD from The RNA Institute at UAlbany.
The large-scale event provided a forum for faculty and students to present their research and network with colleagues, featuring speakers from companies with origins in UAlbany and UR research programs, including Codomax, SupreMEtric, Scriptr, and sxRNA.
The Symposium, hosting ~260 attendees, featured 8 trainee talks, 28 lightning talks, 122 posters from students and postdocs/research faculty, and several invited talks by faculty and industry representatives – including Douglas Anderson, PhD of the Aab CVRI and Scriptr Global and Lynne Maquat, PhD, Director of the Center for RNA Biology and Professor in the Department of Biochemistry & Biophysics. Participants from the UR included 38 graduate students, postdocs, and faculty.
As a co-organizer of this event, Dr. Maquat also hosted the Award Ceremony with Thomas Begley, PhD (Associate Director, The RNA Institute at UAlbany). Details on the student talks and the awards given to UR participants are shown below. The overall Symposium schedule and highlights can be found on the event site: https://www.albany.edu/rna/rna-symposium.
UR Participant Photos
Talks
|
UR Student Talks (8 mins + 2 mins Q&A)
|
|
Lily Cisco
Graduate Student, Lueck Lab, CMPP
|
Benefit of Verapamil on Myotonic Dystrophy Bi-Channelopathy
|
|
Xueyang He
Graduate Student, Boutz Lab, BSCB
|
Modeling the Effects of Cancer-Associated Spliceosome Mutations and Identifying Driving Intronic Features using Deep-Learning Neural Networks
|
|
Madeline (Maddie) Jensen
Graduate Student, Wagner Lab, BMB
|
Identification and Structural Basis of a Novel Licensing Factor of the Integrator Cleavage Module
|
|
UR Student and Postdoc Lightning Talks (2 mins + 1 slide)
|
|
Elizabeth Abshire, PhD
Postdoc, Maquat Lab, DBB
|
AKT Constitutes a Signal-Promoted Alternative Exon-Junction Complex that Regulates Nonsense-Mediated mRNA Decay
|
|
Sara Ali
Graduate Student, Mathews Lab, BSCB
|
Mutate2test: New Algorithm and Software for Mutational Design
|
|
Diego Arévalo
Graduate Student, Miller Lab, CHEM
|
Expanding the Known Structure Space for RNA Binding: A Test of 2,5-diketopiperazine
|
|
Dakarai Esgdaille
Graduate Student, Boutz / Mello Labs, BGG
|
Defining the Regulatory Role of Nuclear Paraspeckles in RNA Splicing in the Context of Pancreatic Tumors
|
|
Justin Galardi
Graduate Student, Kielkopf Lab, BMB
|
HIV-1 Rev Regulates Host Transcription and RNA Processing Factor TatSF1 to Promote HIV-1 Infectivity
|
|
Omar Hedaya
Graduate Student, Yao Lab, BMB
|
Secondary Structures that Regulate mRNA Translation Provide Insights for ASO-Mediated Modulation of Protein Expression
|
|
Wooree Ko
Graduate Student, Lueck Lab, CMPP
|
Anticodon-Engineered Transfer RNA for the Treatment of Nonsense-Associated Cystic Fibrosis
|
|
Adrian Molina Vargas
Graduate Student, O’Connell Lab, BGG
|
Development of New Strategies for the Design of Ultrasensitive Cas13a-Based RNA-Diagnostic Tools with Single-Nucleotide Mismatch Sensitivity
|
Awards
|
Only two total Trainee Speaker Awards were given out, one of which was presented to a UR participant:
|
|
Lily Cisco
Graduate Student, Lueck Lab, CMPP
|
Benefit of Verapamil on Myotonic Dystrophy Bi-Channelopathy
|
|
UR Poster Awards (Recipients of cash awards)
|
|
Hironori (Hiro) Adachi, PhD
Staff Scientist/Postdoc, Yu Lab, DBB
|
Targeted Pseudouridylation: An approach for Suppressing Nonsense Mutations in Disease Genes
|
|
Sara Ali
Graduate Student, Mathews Lab, BSCB
|
Mutate2test: New Algorithm and Software for Mutational Design
|
|
Dakarai Esgdaille
Graduate Student, Boutz / Mello Labs, BGG
|
Defining the Regulatory Role of Nuclear Paraspeckles in RNA Splicing in the Context of Pancreatic Tumors
|
|
Jordana Schmierer
Graduate Student, Takimoto Lab, IMV
|
Host Adaptative Mutations in the pH1N1 PA CTD Affect Genome Replication
|
|
Elinore VanGraafeiland
Graduate Student, Miller Lab, BMB
|
Targeting Programmed Ribosomal Frameshifting in SARS-CoV-2 with a Resin-Bound Dynamic Combinatorial Library
|
|
UR RNA Society Awards (recipients of one-year RNA Society Memberships)
|
|
Elizabeth Abshire, PhD
Postdoc, Maquat Lab, DBB
|
AKT Constitutes a Signal-Promoted Alternative Exon-Junction Complex that Regulates Nonsense-Mediated mRNA Decay
|
|
Wooree Ko
Graduate Student, Lueck Lab, CMPP
|
Anticodon-Engineered Transfer RNA for the Treatment of Nonsense-Associated Cystic Fibrosis
|
|
Jessica Perciaccante
Graduate Student, Anderson Lab, BGG
|
The CARDINAL lncRNA Represses TCF-SRF-Mediated Gene Transcription by Co-Occupying SRF-Bound Gene Promoters in the Heart
|
NYS Center for Excellence in RNA Research and Therapeutics (CERRT). This Symposium reflects an ongoing commitment between the University of Rochester Center for RNA Biology and The RNA Institute at the University at Albany-SUNY to train the next generation of RNA Scientists. This endeavor is occurring in real-time and alongside the NYS Biotech industry to develop an academic pipeline to fuel NYS efforts in harnessing RNA as a therapeutic tool and target.
CALL FOR ABSTRACTS: 2023 Young Investigator Symposium on RNA Biology (hosted by Dr. Xin Li)
Friday, March 10, 2023
 Register (free!) and, postdocs/research-track faculty, submit your abstracts by March 20th: 2023
Register (free!) and, postdocs/research-track faculty, submit your abstracts by March 20th: 2023
2023 Young Investigator Symposium on RNA Biology
Dates: March 24-25, 2023
Venue: VIRTUAL via Zoom Webinar. Please register to obtain the link.
Application Deadline: March 20th, 2023
Register and/or Apply Here (All virtual attendees and talk applicants must register.)
The 2023 Young Investigator Symposium on RNA Biology is co-organized by Xin Li, PhD, Associate Professor at the University of Rochester, the RNA Medical Center of Zhejiang University, and the University of Rochester Center for RNA Biology. The Symposium is free to attend and is focused on talks by dedicated postdocs, instructors, and young (associate) researchers from around the world, in addition to the keynote by Yi-Tao Yu, PhD, Professor of Biochemistry & Biophysics at the University of Rochester. The symposium will feature over 20 scholars, each presenting their cutting-edge research for 30 minutes. It will also feature two career sections on networking and job hunting.
Schedule
Friday, March 24th: Virtual format, opening remarks and then talks, starting at 8:20 am EDT
Saturday, March 25th: Virtual talks and Keynote for a half day, starting at 8:30 am EDT
The symposium will also include two virtual career sections on networking and job hunting. Please see the event website for updates on the program and speakers.
Speakers:
(KEYNOTE) Yi-Tao Yu, University of Rochester
Title: Nonsense suppression induced by RNA-guided RNA modification
Qinqin Cui, Zhejiang University
Title: Diverse CMT2 neuropathies are linked to aberrant G3BP interactions in stress granules
Junnan Fang, Emory University
Title: Isoform expression and the post-transcriptional regulations of centrosome Plp mRNA
Wenxia He, University of North Carolina at Chapel Hill
Title: The Degradation Complex on the 3’ End of Histone mRNA
Yanqiang Li, Harvard Medical School
Title: Low RNA stability signifies increased post-transcriptional regulation of cell identity gene
Ling Liu, University of Manitoba, Canada
Title: Mechanism of Adaptive Splicing Controlled by DNA Methylation
Jianjun Luo, Institute of Biophysics, Chinese Academy of Sciences
Title: Systematic Identification and Functional Studies of Long Noncoding RNAs and SEPs
Yicheng Luo, California Institute of Technology
Title: Maternally inherited siRNAs initiate piRNA cluster formation
Maria Mavrikaki, Harvard Medical School, Beth Israel Deaconess Medical Center
Title: Severe COVID-19 is associated with molecular signatures of aging in the human brain
JingRong Zhao, University of California
Title: Molecular profiling of individual FDA-approved clinical drugs identifies modulators of nonsense-mediated mRNA decay
Zhengjie Zhou, The University of Chicago
Title: Vascular targeted mRNA therapies treating cute Respiratory Distress Syndrome
Please send any questions to Xin Li, PhD
Study: Yi-Tao Yu, Paul Boutz Harness Power, Precision of RNA to Make Mutations Invisible
Monday, March 6, 2023
Scientists have discovered a new way to suppress mutations that lead to a wide range of genetic disorders. A study in the journal Molecular Cell describes a strategy that co-opts a normal RNA modification process within cells to transform disease genes into normal genes that produce healthy proteins. The findings are significant because they may ultimately help researchers alter the course of devastating disorders such as cystic fibrosis, muscular dystrophy and many forms of cancer.
Negating Nonsense
Around 15 percent of mutations that lead to genetic diseases are called nonsense mutations. Aptly named, nonsense mutations occur when an mRNA molecule contains an early “stop” signal. When the mRNA takes genetic instructions from DNA to create a protein, this early stop sign orders the cell to stop reading the instructions partway through the process. This results in the creation of an incomplete protein that can lead to disease.

Led by Yi-Tao Yu, PhD, a team of researchers from the University of Rochester Center for RNA Biology designed an artificial guide RNA – a piece of RNA that can modify other types of RNA – to target mRNA molecules that contain early stop signals (also called premature termination codons). Guide RNAs are a natural mechanism that cells use all the time; Yu’s team altered this already existing process.
Like DNA, RNA is made up of molecular building blocks that are represented by the letters A (adenine), G (guanine), U (uracil), and C (cytosine). Premature termination codons always have the building block U in the first position (for example, UAG, UAA or UGA). The team’s artificial guide RNA was designed to modify the U in the first position, changing the molecular makeup of the targeted mRNA so that the stop signal is no longer – or less well – recognized by the cell.
Read More: Study: Yi-Tao Yu, Paul Boutz Harness Power, Precision of RNA to Make Mutations InvisiblePaul Dunman Elected Fellow of the American Academy of Microbiology
Tuesday, February 28, 2023
The professor of Microbiology and Immunology and Ophthalmology is one of 65 scientists selected for the 2023 fellowship class. Dunman uses Staphylococcus aureus and Acinetobacter baumannii to study how bacteria cause disease, with the goal of developing new therapies to treat bacterial infections.
Read More: Paul Dunman Elected Fellow of the American Academy of MicrobiologyRNA Biologist Lynne Maquat Awarded 2023 Gruber Genetics Prize
Thursday, February 23, 2023
Lynne E. Maquat, PhD, the founding director of the Center for RNA Biology at the University of Rochester, has been awarded the 2023 Gruber Genetics Prize for her discovery of nonsense-mediated mRNA decay or NMD in humans. The Gruber International Prize Program, administered by Yale University, honors scientists from around the world whose groundbreaking work leads to fundamental shifts in knowledge and benefits mankind.
Maquat has spent her career deciphering the many roles that RNA plays in sickness and in health, and is best known for elucidating the complexities of NMD in mammalian cells and human disease. One of the major surveillance systems in the body, NMD protects against mistakes in gene expression by targeting and eliminating deleterious mRNAs that could lead to the production of incomplete and potentially toxic proteins. Maquat’s lab also revealed that NMD helps our cells adjust to changes in development and in their environment, and more rapidly respond to certain stimuli.
“Lynne’s scientific prowess and steadfast commitment to her research is exemplary and has helped catapult the field of RNA biology to the forefront of medicine over the past decade,” said Mark B. Taubman, MD, CEO of the University of Rochester Medical Center and dean of the School of Medicine and Dentistry. “This is an exciting time, as Lynne and other scientists are putting her mechanistic findings related to NMD to use to design treatments. She is incredibly deserving of this honor.”
Read More: RNA Biologist Lynne Maquat Awarded 2023 Gruber Genetics PrizeWork pioneered by Yi-Tao Yu highlighted in new studies
Thursday, February 16, 2023
Rewriting the message: Harnessing RNA pseudouridylation to tackle disease
Matias Montes, Nicole M. Martínez DOI:https://doi.org/10.1016/j.molcel.2023.02.001
Two recent papers in Molecular Cell made strides toward two worthy goals: (1) site-specific modification of RNAs with pseudouridine (Ψ) for functional studies of the epitranscriptome and (2) correction of disease-causing PTCs through Ψ-mediated stop codon readthrough. Both studies build on pioneering work from Yu and colleagues that first showed the potential to engineer small nucleolar RNAs (snoRNAs) for targeted pseudouridylation of stop codons in yeast and discovered that pseudouridylated stop codons suppressed translation termination when artificially introduced in mRNAs.
Read More: Work pioneered by Yi-Tao Yu highlighted in new studiesA serendipitous discovery and the choreographed dance of fragile X research
Tuesday, October 18, 2022
The choreography of development is a delicate dance. Beginning in utero, chromosomes, DNA, genes and RNA twirl, tap, and sashay their way in a precise pattern. A misstep or a missing step that changes the routine causes body and brain functions to go awry – as is the case with many intellectual and developmental disabilities (IDD). Fragile X syndrome is the most common known single-gene cause of inherited IDDs, including autism. Scientists know the misstep in this syndrome is in the gene FMR1. FMR1 is responsible for making the protein FMRP, which is necessary for typical brain development.
Lynne Maquat, Ph.D., founding director of the Center for RNA Biology at the University of Rochester, and professor of Biochemistry & Biophysics, Oncology, and Pediatrics, did not set out to study fragile X. It was through another line of research – her seminal discovery of and decades’ worth of work on nonsense-mediated mRNA decay (NMD) – that fragile X syndrome entered her radar. NMD is a cellular quality-control mechanism that plays a role in both healthy and disease states, and her lab discovered that it is overactive in people with fragile X.
“It was complete serendipity,” Maquat said. “No one ever thought to look at NMD and fragile X. So now we’re trying to figure out what happens at the molecular level when FMRP is absent; we want to understand the network of altered gene expression by identifying mis-regulated messenger RNAs (mRNAs).”
One of the most prominent surveillance systems in the body that protects against mistakes in gene expression that lead to disease, NMD is a complex pathway that is at the heart of many of the collaborations between Maquat and other University of Rochester scientists. Together, with funding from the National Institutes of Health (NIH) and the FRAXA Research Foundation, they aim to gain a deeper understanding of the sophisticated mechanisms related to NMD that will contribute to developing new drug therapies for genetic disorders such as fragile X syndrome, cystic fibrosis, and hundreds of others.
INTO THE BRAIN
Associate professor of Biomedical Genetics Christoph Pröschel, Ph.D., has spent much of his career interested in neurogenetic diseases that primarily affect the white matter of the brain, which carries signals throughout the organ. His lab started working with induced pluripotent stem cells (iPSCs) to understand different neural cell types, providing a solid foundation for their IDD research. “My lab and the Maquat lab have a mutual interest in the molecular mechanism of fragile X,” said Proschel. “It is key to finding any kind of hope for a future therapy.”
The Pröschel lab makes and differentiates neural stem cells that mimic fragile X syndrome, allowing his team to test hypotheses and understand how different therapies impact cell biology and function. He and Tatsuaki Kurosaki, Ph.D., research assistant professor in the Maquat lab, used these neural stem cells to understand the relationship between FMRP and NMD. They discovered that NMD controls the amounts of messenger RNAs deriving from a wide range of genes throughout the brain, including genes that govern motor control and cognitive processes related to attention, learning, and language. They also found that when FMRP is absent from cells, as it is in people with fragile X syndrome, NMD shifts into overdrive.
This work was part of a 2021 study published in Nature Cell Biology led by Maquat that revealed that tamping down NMD with small molecule inhibitors restored a large proportion of neurological functions in these cells.
Most recently, Pröschel co-authored research published in Molecular Cell led by Maquat and co-authored by Hana Cho, Ph.D., and Elizabeth Abshire, Ph.D., of her lab. The study highlighted a complex molecular dance between NMD and the enzyme AKT, which plays a key role in cell growth and survival. Both AKT and NMD are overactive in fragile X. Using neural stem cells that lack the FMRP protein, they tested a drug called Afuresertib, which inhibits AKT. They discovered that blocking AKT in the fragile X cells decreased its activity and decreased NMD. These cells then acted more like typical, non-disease cells.
There is still a lot the team doesn’t know about how AKT and NMD interact, because they both influence and regulate multiple activities in cells, but this work provides good direction that could inform the development of future treatments for fragile X syndrome.
“This has been one of the real fun chapters of my career – working with this group,” said Pröschel. “Everyone brings such a different perspective to the project.”
FROM SURGERY TO THE LAB
As a neurotologist (subspecialist of Otolaryngology), Hitomi Sakano, M.D., Ph.D., spends time in the clinic with patients with hearing issues or hearing loss. In the lab, she aims to understand how the brain adapts to sound information.
Her work with fragile X syndrome began as a resident at the University of Washington when she took interest in FMRP, which is highly expressed in the auditory brainstem nuclei of a typical brain and is the same protein missing in fragile X patients. When Sakano came to the Medical Center in 2018, she brought the fragile X mouse model to study this and joined the Center for RNA Biology.
“I also use the [knockout] mouse model to study hyperacusis – extreme sensitivity to sound,” said Sakano. “We know that fragile X patients have sensory and auditory sensitivity, so this model is a great tool to study both.” Hyperacusis is also very common in the general population (some report up to 15 percent) so understanding the mechanism could potentially impact our broader community.
Sakano hypothesizes that FMRP regulates genes that enable neuroplasticity to maintain
From left: Hitomi Sakano, M.D., Ph.D., and Lynne Maquat, Ph.D.
normal processing of auditory information. If true, there may be therapeutic targets for symptoms like auditory hypersensitivity in fragile X. Funding from the Schmitt Program in Integrative Neuroscience (SPIN) through the Del Monte Institute for Neuroscience Pilot Program and a NIH Research Career Development Award for clinician-scientists are supporting her research, which involves investigating the gene expression abnormalities in the auditory brainstem of the fragile X mouse model that might explain the auditory hypersensitivity in these mice. To date, she has found some interesting RNAs that encode synaptic proteins. These findings open up the possibility of targeting these genes for the treatment of hyperacusis.
She co-authored a study with the Maquat and Pröschel labs in Genome Biology. The research used the mouse model whose FMR1 gene is knocked-out. These findings build upon Maquat’s previous research that showed NMD hyperactivation in neuronally induced stem cells from fragile X patients. This hyperactivity negatively impacts many neuronal mRNAs important to brain development. The Genome Biology paper showed NMD goes into overdrive in the brain during early development in a mouse with fragile X. These researchers are now testing various therapeutics to inhibit NMD.
“Being able to collaborate to gain meaningful results to move this science forward is the value of being at an academic medical center like Rochester,” said Sakano. “These steps are what will ultimately lead to treatments and therapies that I use in the clinic someday to help my patients.”
ON THE HORIZON
Forthcoming research aims to broaden the scope of the fragile X work at the Medical Center. One of the world’s largest clinics for fragile X is in Israel, where an estimated 80 percent of women are screened for the inherited disease. Michael Telias, Ph.D., assistant professor of Ophthalmology, Neuroscience, and Center for Visual Science, began studying fragile X as a graduate student in Israel. He uses human embryonic stem cells that carry the mutation for fragile X to look inside neurons at the molecular and cellular levels to shed light on the human-specific mechanisms affected by this syndrome.
“Human neurons have shown us that these cells have a problem receiving information and communicating information to the next cell,” said Telias. “We cannot do this work in humans, so using human cells enables us to know what to target in the cell. That is the only way we will be able to develop treatments that work.”
In the Frederick J. and Marion A. Schindler Cognitive Neurophysiology Laboratory, research assistant professor Tufikameni Brima, Ph.D., is aiming to use electroencephalography (EEG) and event-related potentials (ERP) to better understand how the brains of patients with fragile X respond to various stimuli. This work has the potential to build upon the ongoing molecular research being conducted by Telias and others.
“Ultimately, what we are figuring out is what happens when FMRP is absent. We don’t know the whole story,” Maquat said. “However, FMRP is an RNA-binding protein, and in work soon to be published in Molecular Cell, Kurosaki and I have now defined those messenger RNAs that are normally bound by FMRP and how the absence of FMRP binding results in those mRNAs making too much protein. These results have allowed us to identify which genes are affected and how. Our work will pave the way for better therapeutics for those living with fragile X.”
Read More: A serendipitous discovery and the choreographed dance of fragile X researchLynne Maquat Receives Advisory Appointment at International Centre for Genetic Engineering and Biotechnology
Friday, August 12, 2022

 Lynne E. Maquat, Ph.D., the J. Lowell Orbison Endowed Chair and Professor of Biochemistry and Biophysics, Oncology and Pediatrics at the University of Rochester School of Medicine & Dentistry has been elected a member of the Council of Scientific Advisers (CSA) of the International Centre for Genetic Engineering and Biotechnology (ICGEB). Also the founding director of the University of Rochester’s Center for RNA Biology, Maquat will serve as a member of the Council for a term of three years, beginning in July 2022.
Lynne E. Maquat, Ph.D., the J. Lowell Orbison Endowed Chair and Professor of Biochemistry and Biophysics, Oncology and Pediatrics at the University of Rochester School of Medicine & Dentistry has been elected a member of the Council of Scientific Advisers (CSA) of the International Centre for Genetic Engineering and Biotechnology (ICGEB). Also the founding director of the University of Rochester’s Center for RNA Biology, Maquat will serve as a member of the Council for a term of three years, beginning in July 2022.
The ICGEB is an intergovernmental organization that runs over 45 state-of-the-art laboratories in Trieste, Italy, New Delhi, India and Cape Town, South Africa. If forms an interactive network with close to 70 member states, and plays a key role in biotechnology by promoting research excellence, training, and technology transfer to industry. The Council of Scientific Advisers is composed of fifteen “eminent” scientists who are active in the life sciences at the international level. Maquat will work together with fellow advisors to provide ICGEB member states with effective training programs and dedicated research projects.
With this appointment, Maquat has held a dozen international advisory positions since 2000. In addition to the ICGEB, she is currently a member of the Scientific Advisory Board for the Max Planck Institute for Molecular Genetics in Berlin, Germany and a member of the Medical Advisory Board for the Canada Gairdner International Awards and The Gairdner Foundation in Canada.
Maquat is the second member of the University of Rochester community to be elected a member of the Council of Scientific Advisers. Arthur Kornberg, M.D., who earned his medical degree from the School of Medicine & Dentistry in 1941 and went on to receive the Nobel Prize in Physiology or Medicine in 1959, served as a scientific advisor to the ICGEB from 1995 to 2005.
Double Duty: Early Research Reveals how a Single Drug Delivers Twice the Impact in Fragile X
Monday, June 27, 2022
Like many neurological diseases, there’s a lot we don’t understand about fragile X syndrome. But, after studying the disorder for several years, Lynne Maquat’s lab knew two important things: the enzyme AKT, which plays a key role in cell growth and survival, and the quality control pathway known as NMD (nonsense-mediated mRNA decay), are both in overdrive in fragile X.
In a new study in the journal Molecular Cell, the team reveals how these two major players interact, highlighting a complex molecular dance that could inform the development of future treatments for fragile X syndrome.
Two paths to pursue
AKT is a hub for cell signaling, helping cells communicate about important processes like cell growth, proliferation and protein production. When cells are stressed – for example, in cancer, diabetes, heart disease and neurological disorders, including fragile X – AKT can send too many (or too few) signals or messages as part of a cell survival mechanism.
NMD is like a molecular guide that helps our cells make smart decisions that (in most cases) improve cellular function and contribute to good health. For example, NMD supports gene expression by flagging and destroying mRNAs (messenger RNAs) that are carrying faulty genetic instructions that could lead to disease. It also helps our cells adjust to changes in development and in their environment, and more rapidly respond to certain stimuli.
Read More: Double Duty: Early Research Reveals how a Single Drug Delivers Twice the Impact in Fragile XConvince us why your favorite RNA or RNA-binding protein is worthy of our admiration
Friday, January 28, 2022
Department of Biochemistry & Biophysics Seminar Series Participants,
Thank you to those who participated in and/or viewed the UR Center for RNA Biology’s RNA Presentations on Jan 12th and 26th, sponsored by the RNA Society, Lexogen, and the UR Center for RNA Biology. The judging committee was impressed with the quality of the abstracts submitted and the selected presentations given by UR graduate students and research staff with the prompt: “Convince us why your favorite RNA or RNA-binding protein is worthy of our admiration”.
Each of the oral presenters, who were chosen based on their quality of their abstracts, will be receiving a one-year membership to the RNA Society. Three presenters will also receive prize funds of $300 each.
The three presenters to be awarded a one-year membership to the RNA Society and $300 ea., in alphabetical order, are:
Xueyang He (presented Jan 12th ) - Biophysics Grad Student, Boutz Lab, Biochemistry & Biophysics
“Modeling the effects of cancer-associated spliceosome mutations and identifying driving intronic features using deep-learning neural networks”
Adrián Moisés Molina Vargas (presented Jan 26th) - Genetics Graduate Student, O'Connell Lab (Biochemistry & Biophysics), Biomedical Genetics
“From prokaryote immunity to the newest RNA targeting tool. Unveiling the nature and opportunities of the Cas13 CRISPR RNA-nuclease”
Li Xie (presented Jan 26th) - Genetics Graduate Student, Pröschel Lab, Biomedical Genetics
“Deciphering eIF2B deficiencies in a neurodegenerative disorder”
Honorable mention presenter to receive a one-year membership to the RNA Society
Perinthottathil Sreejith, PhD (presented Jan 12th ) - Staff Scientist, Bharadwaj Lab, Pathology & Laboratory Medicine, who presented on his previous work as a Postdoc in the Biteau Lab in Biomedical Genetics
“Imp interacts with Lin28 to regulate adult stem cell proliferation in the Drosophila intestine”
Again, thank you all for contributing to make our contest interesting and exciting.
Liz - On behalf of Lynne Maquat, PhD (Director, UR Center for RNA Biology)
Read More: Convince us why your favorite RNA or RNA-binding protein is worthy of our admirationIn the Pocket: RNA Binding Discovery Supports ‘RNA World’ Theory of Early Life on Earth
Friday, January 14, 2022
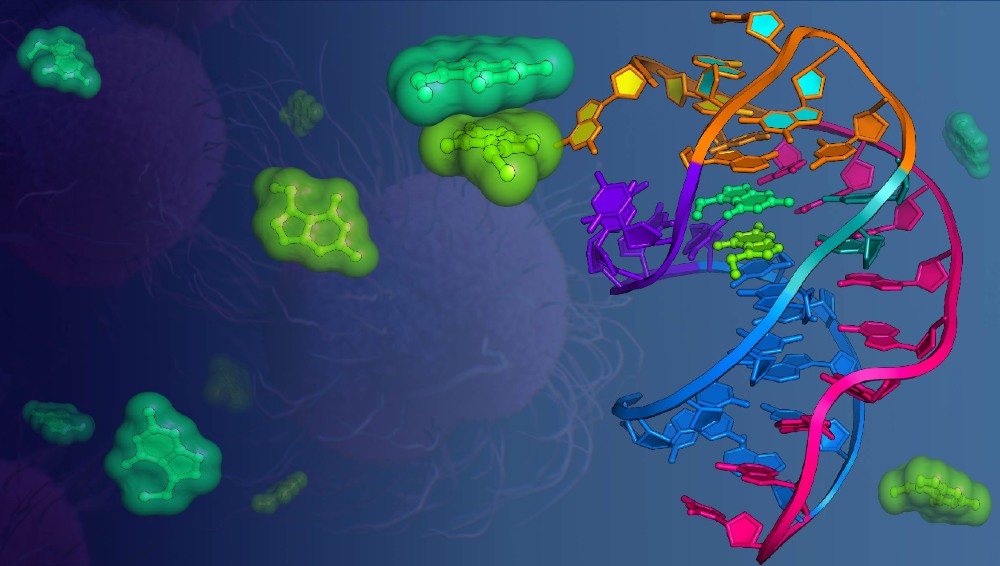

RNA biologists at the University of Rochester Medical Center (URMC) have discovered that RNA, the chemical cousin of DNA, can bind two metabolites (small molecules) at the same time in a single binding pocket, causing those molecules to interact. This discovery, published in Nature Communications this week, could lead to new antibacterial drugs while helping to fill a gap in the controversial “RNA world” theory, which suggests that RNA molecules enabled life to evolve on Earth 3.5 billion years ago.
Read More: In the Pocket: RNA Binding Discovery Supports ‘RNA World’ Theory of Early Life on EarthRNA Presentation Contest: "Convince us why your favorite RNA or RNA-binding protein is worthy of our admiration" - January 2022
Friday, December 3, 2021
The UR Center for RNA Biology is running an RNA presentation contest, sponsored by the RNA Society and Lexogen, for graduate students, postdocs, and lab technicians on the topic: “Convince us why your favorite RNA or RNA-binding protein is worthy of our admiration”
Who is eligible: Any University of Rochester graduate student, postdoc, or lab technician with an interest in RNA biology (No need to be an RNA biologist. Previous contest winners also welcome to apply.)
Entry rules: Contestants for oral presentations will be chosen based on the scientific merit of their abstract (500-word max, excluding title, including references). To be considered for the oral competition, abstracts conforming to the rules, detailed below, must be submitted by 12pm on January 3rd.
Prizes: Three prizes of a one-year membership in the RNA Society plus $300 will be given to each of the three top oral presenters. Those who are already RNA Society members will be given membership for the following year. The prize funds should be used toward attending a research meeting.
Details: Contestants for oral presentation will be selected by an anonymous panel of faculty judges based on the scientific merit of their submission (500 words or fewer). In addition to the ~500-word description, please include your name, department, lab, status, year(s) at the UR, and if you were or are supported by a fellowship.
Those selected for the oral competition will give a 2-min intro of themselves, and an 8-minute science talk about their favorite RNA or RNA-binding protein followed by a 3-min Q&A session. Presentations will January 12th and 26th during the usual Department of Biochemistry & Biophysics Seminar Series on Wednesdays at 2pm (virtual or in-person TBD). Applicants and contestants should plan to be present for both January dates. Winners will be determined by the judges based on the quality of their presentations and their ability to address questions during the Q&A session.
Email submissions by 12pm on January 3rd to elizabeth_leverenz@urmc.rochester.edu.
Oral contestants will be notified by January 5th.
View the flyer
Lynne Maquat spoke with WXXI: 'A wave of the future': local biologist explores what's next for mRNA technology
Wednesday, September 8, 2021
The recent approval of Pfizer and BioNTech’s COVID-19 vaccine by the U.S. Food and Drug Administration, has scientists enthusiastic about the future of the messenger RNA, or mRNA, technology used to produce the vaccine. For RNA biologists, in particular, the pandemic has become a catalyst for modern medicine. “It's a big step forward for mRNA-based therapeutics,” said UR Medicine’s director for centers for RNA biology, Dr. Lynne Maquat. “We've had a worldwide need. And the need has been met.”
Maquat defines mRNA as the messenger molecule between our DNA, and the proteins that our cells need to perform their duties. She says the COVID-19 vaccine produces a piece of the spike protein that surrounds the coronavirus and raises an immune response to it.
“Our immune system will attack the virus, because the spike protein is on the outside of the virus,” said Maquat.
She says the success of the Pfizer and Moderna vaccines is causing scientists to look at other illnesses that could benefit from the mRNA technology like dengue.
“There's lots of infectious diseases, especially in Third World countries that need to be addressed,” Maquat said.
UR Medicine infectious disease specialist Dr. Edward Walsh says existing vaccines can also benefit from messenger RNA.
“If an mRNA vaccine for the influenza vaccine could reach the kind of efficacy data, especially over a six-month period, we really only need to get protection for about six months through the flu season each year,” Walsh said.
He said the flu vaccine is roughly 60% effective, but mRNA technology could increase that...
“If we could get that very high degree of efficacy, it could really do a lot of public health good,” said Walsh.
Maquat said she and her team are already looking at how some cancers could be treated with messenger RNA technology
“Let's say you have a pancreatic cancer,” said Maquat, “you could have your tumor removed, and you can have it characterized for new proteins.”
The new discovered proteins in the tumor would allow scientists to develop an mRNA treatment to combat that protein.
“It's a type of personalized medicine,” said Maquat. "This is a wave of the future"
Read More: Lynne Maquat spoke with WXXI: 'A wave of the future': local biologist explores what's next for mRNA technologyCongratulations Omar Hedaya
Tuesday, August 31, 2021
 Congratulations to Omar on receiving his Poster Award from the 26th RNA Society Annual Conference in August 2021!
Congratulations to Omar on receiving his Poster Award from the 26th RNA Society Annual Conference in August 2021!
Hooray!
Congratulations Dr. Wu
Wednesday, August 25, 2021

Congratulations on Dr. Jiangbin Wu's receiving the Career Development Award from the American Heart Association that started on July 1st, 2021 and being selected as a finalist of the Outstanding Early Career Investigator Award for the BCVS2021 Scientific session!
His talk presentation occurred at 3:15-3:30PM on 8/24/2021.
That's the Rochester Effect: Featuring Dr. Lynne Maquat. PhD
Friday, July 2, 2021
Lynne Maquat Wins Warren Alpert Foundation Prize
Monday, May 3, 2021
Lynne Maquat, Ph.D., the founding director of the Center for RNA Biology at the University of Rochester, has been awarded the Warren Alpert Foundation Prize for her pivotal discoveries in the field of RNA biology. She shares the prize with fellow RNA biologist Joan Steitz, Ph.D., Sterling Professor of Molecular Biophysics and Biochemistry at Yale School of Medicine.
The award is given by the Warren Alpert Foundation in recognition of work that has improved the understanding, prevention, treatment or cure of human disease. The prize is administered by Harvard Medical School, and since its inception in 1987, 12 honorees have gone on to receive Nobel prizes.
George Q. Daley, dean of Harvard Medical School, said of Maquat and Steitz: "The discoveries made by the two award winners are stunning in their elegance and scope." Earlier this year, the pair won the 2021 Wolf Prize in Medicine for their work. They shared the Wolf Prize with a third RNA biologist, Adrian Krainer, Ph.D., of Cold Spring Harbor Laboratory.
RNA Makes its Mark
Maquat has studied RNA since she started her own laboratory in 1982. But until the development and approval of multiple mRNA COVID-19 vaccines in 2020, RNA wasn't on the public radar. Decades of research by Maquat and Steitz on how RNAs work and how they are involved in human disease helped set the stage for the rapid development of these vaccines, which are key to bringing the COVID pandemic under control.
Many are familiar with DNA, which contains the genetic instructions that make us who we are. But RNA is equally important in the process of gene expression:
- DNA lives in the nucleus of a cell and makes many types of RNA, including mRNA (messenger RNA).
- mRNA takes genetic instructions from DNA, travels out of the nucleus and delivers them to factories in our cells called ribosomes.
- Ribosomes use the instructions to create proteins.
- Proteins carry out myriad functions throughout the body, including ferrying nutrients around, helping with chemical reactions and protecting us from disease.
Many disorders result from problems with our DNA, but over the past several decades Maquat, Steitz and other scientists revealed that mRNA plays a role in a multitude of diseases, too. Cystic fibrosis, fragile X syndrome, Duchene muscular dystrophy and a long list of other inherited and acquired diseases result from defective mRNAs.
"Scientists used to think that gene regulation was all about DNA, and that RNA didn't influence gene expression, the production of proteins or the development of disease," said Maquat, the J. Lowell Orbison Endowed Chair and Professor of Biochemistry and Biophysics, Oncology and Pediatrics at the University of Rochester School of Medicine and Dentistry. "We now know that a very complicated network exists in our cells to regulate how, when and where our mRNAs are produced. This knowledge has opened the door to using RNA as both a treatment target and a tool to develop new therapies."
Lynne Maquat Awarded 2021 Wolf Prize in Medicine
Tuesday, February 9, 2021
Lynne E. Maquat, Ph.D., the founding director of the Center for RNA Biology at the University of Rochester, was honored with the 2021 Wolf Prize in Medicine. The acclaimed international award is given to outstanding scientists from around the world for achievements that benefit mankind.
Maquat was selected for "fundamental discoveries in RNA biology that have the potential to better human lives." She has spent her career deciphering the many roles that RNA plays in sickness and in health, and is well known for her discovery of nonsense-mediated mRNA decay or NMD. One of the major surveillance systems in the body, NMD protects against mistakes in gene expression that lead to disease. Maquat's lab also revealed that NMD helps our cells adjust to changes in development and in their environment, and more rapidly respond to certain stimuli.
Maquat shares the award with Joan Steitz, Ph.D., Sterling Professor of Molecular Biophysics and Biochemistry at Yale School of Medicine and Adrian Krainer, Ph.D., St. Giles Foundation Professor and Cancer Center Deputy Director of Research at Cold Spring Harbor Laboratory. Steitz and Krainer were also honored for discoveries in RNA biology.
The Wolf Foundation, which celebrates exceptional achievements in the sciences and the arts, is based in Israel, where Maquat's quest to unravel the intricacies of NMD began. In 1980 she traveled to Jerusalem to retrieve bone marrow samples from four children suffering from thalassemia major, the most severe form of the inherited blood disorder thalassemia. Maquat wanted to learn why the children's marrow contained no beta-globin protein, which is necessary for the oxygen-carrying function of red blood cells. Her 1981 breakthrough manuscript, "Unstable beta-globin mRNA in mRNA-deficient beta0 thalassemia," published in Cell, was the first to reveal the role of NMD in human cells and how it can lead to disease.
"Lynne's work on nonsense-mediated mRNA decay is the bedrock of an ever-growing body of research on how mRNAs are monitored and regulated," said Mark B. Taubman, M.D., dean of the University of Rochester School of Medicine and Dentistry. "Her dedication to her science and to the field of RNA biology has opened the door to the development of RNA-based therapeutics for a wide range of disorders that you can't reach with conventional drugs. We're thrilled that her contributions are being recognized with this prestigious award."
RNA secured its place in the public eye in 2020 with the development and approval of multiple mRNA COVID-19 vaccines. Years of research by Maquat, Steitz and Krainer helped set the stage for the rapid development of these vaccines.
The J. Lowell Orbison Endowed Chair and Professor in the Department of Biochemistry and Biophysics at the University of Rochester School of Medicine and Dentistry, Maquat is the recipient of several other significant honors, including:
Winners of the Wolf Prize are selected annually by an international jury committee of the Wolf Foundation; prizes are awarded regardless of religion, gender, race, geographical region, or political view. The official announcement of this year's prize by the President of the State of Israel, Reuven Rivlin, was made on February 9, 2021.
UR Center for RNA Biology RNA Presentation Contest Results
Tuesday, February 2, 2021
Thank you to those who participated in and/or viewed the UR Center for RNA Biology's RNA Presentations on Jan 6th, 13th, and 27th, sponsored by the RNA Society and Lexogen. The judging committee was impressed with the quality of the presentations given on all three dates, making final decisions difficult.
All nine of the graduate student, postdoc, and technician presenters, chosen based on their quality of their abstract, will be receiving a one-year membership to the RNA Society. Of these, four who were chosen based on the quality of their presentation will be awarded $375 each to be used toward the attendance of an RNA-centric meeting, in person or virtually.
The four to be awarded a one-year membership in the RNA Society and $375, in alphabetical order, are:
Kamel Awayda (presented Jan 6th )
Technician, Mitch O'Connell Lab
"Characterization of THUMP Domain-Containing Protein 1, and Putative Modulator of RNA Modification"
Chen Bao (presented Jan 13th)
Biochemistry Grad Student, Dmitri Ermolenko Lab
"mRNA Stem-Loops Can Induce Translational Pause through Two Pathways"
Xavier Rambout, PhD (presented Jan 27th)
Postdoc, Lynne Maquat Lab
"Transcriptional Coactivator PGC-1α Contains a Novel CBP80-Binding Motif that Orchestrates Efficient Target-Gene Expression"
Arica VanderWal (presented Jan 13th)
Biochemistry Grad Student, Mitch O'Connell Lab
"The Role of Csx28 in CRISPR-Cas13b Anti-Bacteriophage Defense"
The remaining to be awarded a one-year membership in the RNA Society, in alphabetical order, are:
Lily Cisco (presented Jan 6th )
Pharm-Phys Grad Student, John Lueck Lab
"Testing Therapeutic Intervention for Myotonic Dystrophy Type 1 in a Novel Mouse Model"
Xueyang He (presented Jan 6th )
Biophysics Grad Student, Paul Boutz Lab
"A Novel Method to Capture and Sequence Intron Lariats"
Shon Koren (presented Jan 6th )
Technician, Gail Johnson Lab
"Tau-Ribosome-tRNA Associations Underlie Common Features of Dys sincening Protein Synthesis in Dementia"
Sean Lindley (presented Jan 13th )
Biology Grad Student, Doug Anderson Lab
"StitchR: Ribozyme-Mediated RNA Trans-Splicing and Expression in Mammalian Cells"
Debanjana Maji (presented Jan 27th )
Biophysics Grad Student, Clara Kielkopf Lab
"Representative Cancer-Relevant U2AF2 Mutations Alter RNA Interactions and Splicing"
Beneath the Surface of Fragile X Syndrome: Study Sheds Light on What’s Happening in Nerve Cells
Monday, January 25, 2021
A new study shows that many abnormalities in fragile X syndrome cells are related to glitches with one of the body's major quality control systems. Published in Nature Cell Biology, the research provides fresh insight into the molecular mechanisms of the disorder and a pathway to search for potential treatments.
Fragile X syndrome occurs when individuals don't make the fragile X protein known as FMRP, which is needed for normal brain development. Currently, little is known about how the loss of this crucial protein leads to the intellectual disability and severe learning problems characteristic of the disease.
Rochester researchers found that many irregularities in cells that lack FMRP are due to misregulated nonsense-mediated mRNA decay, or NMD. Discovered by RNA biologist Lynne E. Maquat, Ph.D., NMD is like a molecular guide that helps our cells make smart decisions that (in most cases) improve cellular function and contribute to good health. For example, NMD helps our cells adjust to changes in development and in their environment, and more rapidly respond to certain stimuli.
Through a series of cellular analyses, Maquat's team discovered that NMD influences a wide range of genes throughout the brain, including genes that govern motor control and cognitive processes related to attention, learning and language. They also found that when FMRP is absent from cells, as it is in people with fragile X syndrome, nonsense-mediated mRNA decay shifts into overdrive.
New Research Funding Initiative to Support Young Investigators
Friday, January 15, 2021
Thanks to a partnership between pediatric researchers and the GCH Advancement division, a new funding initiative will seek to provide $25,000 -- matched with grant funds -- to support individual young investigators who are undertaking pediatric research.
These young investigators would be tenure-track assistant professors or post-doctoral fellows who already have a PhD degree and are on the path to obtain a tenure track position. This type of "seed" funding will be critical for fostering the careers of young researchers at URMC and could potentially pay dividends for institutional growth in the long-term, according Tom Mariani, PhD, professor of Neonatology and director of research for the Department of Pediatrics.
"In addition to funding discovery and innovation which could potentially save millions of lives, the career development of one investigator can be leveraged many times over to merit additional external funding, creation of new labs, and stimulate economic growth in the Rochester community."
Alan Wood, owner of the Realty company RE/Max Plus and supporter of GCH, and Lynne Maquat, PhD, endowed chair and professor in the Departments of Biochemistry and Biophysics, Oncology, and Pediatrics, developing this funding idea after Wood and his children visited Maquat's Fragile X Syndrome lab this Fall. Fragile X Syndrome is the most common single-gene cause of autism and intellectual disability, and Wood's family got a thorough education on the scope of Maquat's work, which is examining disease-causing mutations in children, and what those mutations do to gene expression via RNA production.
"My children loved getting first-hand experience touring the lab and seeing real-world visualizations of the work Maquat and her team are doing. Wearing lab coats and gloves, they isolated genetic material from bananas, which turn out to have more genetic material than us humans," said Wood.
Wood will be leading the effort to develop the program and secure funding. Donors who sponsor a young investigator will be able to establish a relationship to learn more about their research as it evolves.
"We have several talented young researchers in the Department of Pediatrics," said Maquat, "providing early support for their career is a long-term goal. The results won't be immediate, but over time, this support will build the foundation for discovery that will improve kids' lives."
New Research Findings Published in 2020 from Yao Lab
Wednesday, December 30, 2020
Cardiovascular disease (CVD is the number one killer of human beings and have recently resulted even more human death in combination with COVID19 virus infection during the pandemic. As exemplified by the two recently FDA approved mRNA vaccines from Pfizer and Moderna companies, we are so excited that mRNA plus translation can make our own cells manufacture vaccine proteins to protect us from virus infections and relieve the burden for our cardiovascular system. Importantly, mRNAs can also produce therapeutic proteins via the translation process to fight against a variety of diseases including cardiovascular disease and cancer. Our research perfectly aligns with the goal and missions of these pharmaceutical companies in aiming to utilize or manipulate mRNA translation to treat human diseases.
In July 2020, we published our results in Circulation Research [1] about the function of an evolutionarily conserved, house-keeping enzyme glutamyl-prolyl-tRNA synthetase in cardiac fibrosis, and a Chinese medicine derived compound halofuginone in antagonizing fibrosis via inhibiting proline-rich protein translation. This compound was used for treating malaria infection and arrhythmia in ancient China for more than 2000 years. High dose of the drug can inhibit global protein synthesis via blocking the incorporation of proline amino acid, a building block of mRNA translation. Our data suggests that low dose of this drug actually can provide therapeutic benefit in heart disease treatment by mildly inhibiting translation of proline-rich proteins, which are mostly encoded by pro-fibrotic extracellular matrix transcripts in cardiac fibroblasts. Selective repression of expression of these fibrogenic proteins by knocking out one allele of Eprs gene in loss-of-function genetic mouse models or inhibiting enzymatic activity of EPRS protein, remarkably reduces cardiac fibrosis and hypertrophy, and partially restored cardiac function in heart failure mouse models.
On December 28th 2020, we had another paper published online in EMBO Molecular Medicine [2] showing that a cardioprotective small noncoding RNA, microRNA-574 (miR-574), plays important role in maintaining mitochondrial translation homeostasis and normal cardiac function via fine-tuning the expression of a novel mitochondrial translation regulatory factor FAM210A (family with sequence similarity 210 member A). Mitochondria are the power houses of living cells. They provide ATP as energy currency to support any biochemical reactions in our own body. Dysfunction of mitochondria caused by aberrant expression of mitochondrial proteins is known to trigger severe mitochondrial cardiomyopathy and heart failure. In this work, we used an miR-574 genetic knockout mouse model and transcriptome-wide gene expression profiling to discover that miR-574-FAM210A axis plays a critical role in maintaining optimal mitochondrial protein translation and protecting heart functions under cardiac stress conditions.
To confirm the relevance of EPRS and miR-574 in human heart failure, we collaborated with a cardiologist and physician scientist Dr. Wai Hong Wilson Tang from Cleveland Clinic to obtain human failing heart and non-failure tissue samples to measure the expression of EPRS and miR-574 and validated their relationship with human heart disease. A major challenge in these studies is to define the downstream molecular targets of EPRS and miR-574. With the extensive collaborative efforts from the University of Rochester Genomics Core, we performed the polysome profiling coupled with next generation RNA deep sequencing for halofuginone-treated, EPRS-inhibited fibroblast cells, and RNA-sequencing for miR-574 knockout mouse hearts, respectively. We successfully identified the target mRNAs of EPRS and miR-574 and discovered a number of novel therapeutic targets (such as SULF1 and FAM210A, respectively) for treatment of cardiac fibrosis and pathological remodeling.
- Jiangbin Wu, Kadiam C Venkata Subbaiah, Li Huitong Xie, Feng Jiang, Eng-Soon Khor, Deanne Mickelsen, Jason R. Myers, Wai Hong Wilson Tang, and Peng Yao. Glutamyl-prolyl-tRNA synthetase regulates proline-rich pro-fibrotic protein synthesis during cardiac fibrosis. Circulation Research 2020 127:827–846
- Jiangbin Wu, Kadiam C Venkata Subbaiah, Feng Jiang, Omar Hedaya, Amy Mohan, Tingting Yang, Kevin Welle, Sina Ghaemmaghami, Wai Hong Wilson Tang, Eric Small, Chen Yan, and Peng Yao. MicroRNA-574 regulates FAM210A expression and influences pathological cardiac remodeling. EMBO Molecular Medicine Online published (https://doi.org/10.15252/emmm.202012710)
Blog: Are the new mRNA vaccines safe?
Wednesday, December 30, 2020

John Lueck
Joanne Ladolcetta, a freelance writer based out of San Francisco, wanted to put together information to help address circulating fears and misconceptions about the recently FDA approved COVID-19 mRNA vaccines. She reached out to friends Bryant Johnson (Cartoonist) and John Lueck (Researcher at the University of Rochester Medical Center) to tackle some of the most common vaccine questions. The collaboration resulted in an approachable informative write-up with creative illustrations and helpful links to provide readers the ability to do a deeper dive into the generation and application of the novel COVID-19 mRNA vaccines.

Courtesy of Bryant Johnson
Read More: Blog: Are the new mRNA vaccines safe?
University of Rochester Center for RNA Biology Presentation Contest, January 2021
Wednesday, December 23, 2020
RNA Presentations by UR Grad Students, Postdocs, and Techs!
Sponsored by the RNA Society, Lexogen, and the UR Center for RNA Biology
Virtual presentations will be on Wednesdays at 2pm EST on January 6th, 13th, & 27th during the usual Department of Biochemistry & Biophysics Seminar. There will be 3-4 presentations on each date (~20-min slots). Winners will be determined by the anonymous panel of judges based on the quality of their lay and research presentations and their ability to address questions during the Q&A session.
Register Here
JANUARY 2021 PRESENTATION SCHEDULE
Day 1: Wed, Jan 6th
2:00 -- 3:20pm
|
2:00pm
|
Lily Cisco, Lueck lab, Pharm-Phys grad student
|
|
2:20pm
|
Xueyang He, Boutz lab, Biophysics grad student
|
|
2:40pm
|
Shon Koren, Johnson lab, technician
|
|
3:00pm
|
Kamel Awayda, O'Connell lab, technician
|
Day 2: Wed, Jan 13th
2:00 -- 3:00pm
|
2:00pm
|
Chen Bao, Ermolenko lab, Biochemistry grad student
|
|
2:20pm
|
Sean Lindley, Anderson lab, Biology grad student
|
|
2:40pm
|
Arica VanderWal, O'Connell lab, Biochemistry grad student
|
Day 3: Wed, Jan 27th
2:00 -- 3:00pm
|
2:00pm
|
Deb Maji, Kielkopf lab, Biophysics grad student
|
|
2:20pm
|
Elizabeth Abshire, PhD, Maquat lab, postdoc
|
|
2:40pm
|
Xavier Rambout, PhD, Maquat lab, postdoc
|
Contestants for oral presentations were selected based on the scientific merit of their abstract submissions, in December, by an anonymous panel of six faculty judges and two graduate student judges. Competitors will present a 2-min lay talk without slides followed by a 12-min slide presentation of their research, i.e. the topic of their abstract, and a 3-min Q&A session.
COVID-19 vaccine: What’s RNA research got to do with it?
Monday, December 14, 2020
The US Food and Drug Administration recently approved emergency use authorization for a COVID-19 vaccine developed by Pfizer and the German pharmaceutical company BioNTech.
The vaccine made history not only because it reported a 95 percent efficacy rate at preventing COVID-19 in clinical trials, but because it is the first vaccine ever approved by the FDA for human use that is based on RNA technology.
"The development of RNA vaccines is a great boon to the future of treating infectious diseases," says Lynne Maquat, the J. Lowell Orbison Distinguished Service Alumni Professor in biochemistry and biophysics, oncology, and pediatrics at Rochester and the director of Rochester's Center for RNA Biology.
COVID-19, short for "coronavirus disease 2019," is caused by the novel coronavirus SARS-CoV-2. Like many other viruses, SARS-CoV-2 is an RNA virus. This means that, unlike in humans and other mammals, the genetic material for SARS-CoV-2 is encoded in ribonucleic acid (RNA). The viral RNA is sneaky: its features cause the protein synthesis machinery in humans to mistake it for RNA produced by our own DNA.
For that reason, several of the leading COVID-19 vaccines and treatments are based on RNA technology.
A contingent of researchers at the University of Rochester study the RNA of viruses to better understand how RNAs work and how they are involved in diseases. This RNA research provides an important foundation for developing vaccines and other drugs and therapeutics to disrupt the virus and stop infections.
"Understanding RNA structure and function helps us understand how to throw a therapeutic wrench into what the COVID-19 RNA does—make new virus that can infect more of our cells and also the cells of other human beings," Maquat says.
In the past few decades, as scientists came to realize that genetic material is largely regulated by the RNA it encodes, that most of our DNA produces RNA, and that RNA is not only a target but also a tool for disease therapies, "the RNA research world has exploded," Maquat says. "The University of Rochester understood this."
In 2007, Maquat founded The Center for RNA Biology as a means of conducting interdisciplinary research in the function, structure, and processing of RNAs. The Center involves researchers from both the River Campus and the Medical Center, combining expertise in biology, chemistry, engineering, neurology, and pharmacology.
Cool Technology Allows for Better Views of Cancerous Blood Cells in Quest for New Treatment
Tuesday, September 15, 2020

Clara Kielkopf, Ph.D., left, and Laura Calvi, M.D., stand by the University's new cryo-microscope
With the recent acquisition of Nobel Prize-winning technology and two new grants, Wilmot Cancer Institute researchers are streamlining their investigations into a malignant blood disease known as MDS, working toward discovering targeted therapies.
Laura Calvi, M.D., and Clara Kielkopf, Ph.D., are leading collaborative teams that will be using a device at the University of Rochester Medical Center — a cryo-electron microscope — that has ushered in a new era in biochemistry. The microscope allows scientists to see 3D snapshots and more details of living molecules than ever before, down to near-atomic resolution, to understand disease and uncover new ways to design drugs. The developers of the technology were awarded the Nobel Prize in Chemistry 2017.
Calvi and Kielkopf each received Edward P. Evans Foundation awards totaling $1.2 million for this project. Evans grants go to scientists seeking a cure for myelodysplastic syndromes (MDS), which originates in the bone marrow and disrupts healthy blood cell formation. MDS often leads to leukemia.
The Kielkopf lab will use the cryo-electron microscope to obtain 3D views of recurrent MDS mutations as guides for targeting molecular therapies. The modern microscope is the first of its kind in the Rochester region, Kielkopf said, and will be accessible to all UR researchers through the Electron Microscopy Shared Resource Laboratory. She is a professor of Biochemistry and Biophysics in the Center for RNA Biology.
David Mathews, MD, PhD installed as first Lynne Maquat Distinguished Professor in RNA Biology
Tuesday, September 15, 2020
We are thrilled to announce that Professor David Mathews, MD, PhD, was honored at this year's School of Medicine Opening Convocation by being installed as the first Lynne Maquat Distinguished Professor in RNA Biology in the School of Medicine and Dentistry. The professorship was awarded by Dr. Mark Taubman, Dean of the School of Medicine and CEO of the University of Rochester Medical Center. The Convocation was held virtually this year and can be viewed at the link below.
Congratulations to Professor Mathews!
RNA Essay Contest Results and Congratulations
Wednesday, August 26, 2020
The UR Center for RNA Biology offered an exercise during the time when COVID-19 became a sufficient threat to largely shut-down our research enterprise. We're pleased to announce the winners of the UR's Center for RNA Biology Essay Contest on "The role of RNA research in community health". These awards are sponsored by a grant from the RNA Society & Lexogen to the UR Center for RNA Biology, and funds from UR RNA Structure & Function Cluster.
Our Gold prize (~$1,000 value) award goes to Sydney Simpson, an Immunology, Microbiology & Virology graduate student in Steve Dewhurst's lab in the Department of Microbiology & Immunology, for her essay: "Nucleoside Analog Inhibitors: Timeless & Timely Beacons of Hope".
The Silver Prize (~$250 value) award goes to Omar Hedaya, a Biochemistry & Molecular Biology graduate student in Peng Yao's lab in the Department of Medicine/Department of Biochemistry & Biophysics, for his essay: "Know the Fundamentals when Seeking the Future".

Omar Hedaya

Sydney Simpson
Both awardees have become members of the RNA Society and will use their winnings toward technology needs for the upcoming semester.
The RNA Society now features our contest results, including the winning essays, in its latest RNA Salon update: https://www.rnasociety.org/featured-salons, see bullet #3.
We would like to acknowledge Honorable Mentions for the following applicants:
- Sai Shashank Chavali -- Graduate student; Biophysics, Structural & Computational Biology; Wedekind Lab
- Lily Cisco -- Graduate student; Cellular and Molecular Pharmacology & Physiology; Lueck Lab
- Gabrielle Kosoy -- Graduate student; Biophysics, Structural & Computational Biology; Miller Lab
- Ashwin Kumar -- Graduate student; Biophysics, Structural & Computational Biology; Topham Lab
- Li Xie -- Graduate student; Genetics, Development & Stem Cells; Pröschel Lab
We thank all who participated -- and our judges, too!
Kielkopf Lab Awarded EvansMDS Research Grant
Thursday, July 16, 2020
Prof. Clara Kielkopf has been awarded an EvansMDS research grant to use the new, state-of-the-art cryo-electron microscope at the U of R Medical Center EM facility. The Kielkopf group will use this revolutionary technique to study 3D structures of mutant U2AF1-splicing complexes in human malignancies. The title of the EvansMDS grant is: "Cryo-Electron Microscopy Structures of Mutant U2AF1-Containing Ribonucleoproteins Associated with Myelodysplastic Syndromes". This grant was made possible by our new Talos cryo-EM.
This week’s URMC Research Heroes featured the Maquat lab’s Tatsuaki Kurosaki, PhD, and Shuhei Mitsutomi, MS
Wednesday, June 3, 2020
This week's URMC Research Heroes featured the Maquat lab's Tatsuaki Kurosaki, PhD, and Shuhei Mitsutomi, MS, who were recognized today for their work on SARS-CoV-2.
https://www.instagram.com/p/CA-QN7oAl07/
Both Tatsuaki and Shuhei have worked as members of the Maquat Lab (Center for RNA Biology and the Department of Biochemistry & Biophysics) during the sequestration on SARS-CoV-2, collaborating with a lab at Harvard to determine the mechanism by which the virus inhibits human-cell nonsense-mediated mRNA decay (NMD) so as to express and replicate its RNA efficiency.
From Tatsuaki: "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease 19 (COVID-19) pandemic, is a novel enveloped RNA virus carrying a large (~30 kb) positive-sense single-stranded RNA genome. Although human cells innately have an RNA surveillance pathway called nonsense-mediated mRNA decay (NMD) that generally protects cells from infection by many different types of viruses, little is known about how SARS-CoV-2 inhibits NMD to proliferate in human cells. We hope that our research helps to elucidate the molecular mechanisms of SARS-CoV-2 proliferation in human cells, eventually contributing toward the development of therapeutic strategies to combat COVID-19."
University of Rochester RNA Essay Contest: “The role of RNA research in community health”
Wednesday, May 20, 2020
Sponsored by the RNA Society & Lexogen, UR RNA Structure & Function Cluster, and UR Center for RNA Biology
Who is eligible: Any University of Rochester graduate student or post-doc with an interest in RNA biology
Entry rules: Essays should be no more than two pages, single-spaced (excluding references, which should be present), 11-point Arial font, with half-inch margins all around.
Prizes: Two prizes will be given out. Gold (valued at ~$1000), and Silver (valued at ~$250). Additionally, winning essays along with photos of the winning authors will be posted on the Center for RNA Biology webpage and featured on the RNA Society's RNA Salon page, offering international exposure.
Details
The UR's Center for RNA Biology is running an essay contest, sponsored by the RNA Society & Lexogen, and UR's RNA Structure & Function Cluster, on "The role of RNA research in community health". This contest, which is open to all UR graduate students and post-docs, aims to promote creative yet data-driven thinking about the importance of RNA in the "big picture".
Considering that reliable technology is required for research in an increasingly virtual world, prizes will consist of a PC or Mac laptop for Gold winners (~$1000), and software licenses or peripherals (e.g., second monitor or laptop dock) for Silver winners (~$250), subject to the needs of each recipient.
Submissions must be emailed to Liz by Monday, July 13th, 2020.
Winners will be announced in the beginning of August.
Links
The RNA Society: https://www.rnasociety.org/
RNA Structure & Function Cluster: http://www.rochester.edu/ucis/rnastructure.html
Center for RNA Biology: https://www.urmc.rochester.edu/rna-biology.aspx
Installation of a new Talon L210C cryo-EM facility
Friday, May 15, 2020
Center for RNA Biology Contributes to Fighting Coronavirus
Tuesday, April 28, 2020
Viruses like the coronavirus that causes COVID-19 are able to unleash their fury because of a devious weapon: ribonucleic acid, also known as RNA.
A contingent of researchers at the University of Rochester study the RNA of viruses to better understand how RNAs work and how they are involved in diseases. As COVID-19 continues to spread around the globe, RNA research provides an important foundation for developing antiviral drugs, vaccines, and other therapeutics to disrupt the virus and stop infections.
"Understanding RNA structure and function helps us understand how to throw a therapeutic wrench into what the COVID-19 RNA does—make new virus that can infect more of our cells and also the cells of other human beings," says Lynne Maquat, professor of biochemistry and biophysics at the University of Rochester Medical Center and the director of Rochester's Center for RNA Biology.
In the past few decades, as scientists came to realize that genetic material is largely regulated by the RNA it encodes, that most of our DNA produces RNA, and that RNA is not only a target but also a tool for disease therapies, "the RNA research world has exploded," Maquat says. "The University of Rochester understood this."
In 2007, Maquat founded the Center for RNA Biology as a means of conducting interdisciplinary research in the function, structure, and processing of RNAs. The center involves researchers from both the River Campus and the Medical Center, combining expertise in biology, chemistry, engineering, neurology, and pharmacology.
While much of the research across the University has been put on pause, labs that are involved in coronavirus research remain active.
"Our strength as a university is our diversity of research expertise, combined with our highly collaborative nature," says Dragony Fu, an assistant professor of biology on the River Campus and a member of the Center for RNA Biology. "We are surrounded by outstanding researchers who enhance our understanding of RNA biology, and a medical center that provides a translational aspect where the knowledge gained from RNA biology can be applied for therapeutics."
Lynne Maquat Honored by International Union of Biochemistry and Molecular Biology
Tuesday, October 29, 2019
Lynne Maquat, Ph.D., the J. Lowell Orbison Endowed Chair and Professor in the Department of Biochemistry and Biophysics, was honored with the International Union of Biochemistry and Molecular Biology (IUBMB) Jubilee Lectureship in September. The IUBMB unites biochemists and molecular biologists in 75 countries and is devoted to promoting research and education in biochemistry and molecular biology, giving particular attention to areas where the subject is still in its early development.
The IUBMB Jubilee Lectureship was established to commemorate the 50th anniversary of the First International Congress of Biochemistry that was held in Cambridge, England in 1949, at which the initial steps were taken that led to the formation of IUBMB. Maquat gave the keynote lecture at the IUBMB Molecular Biosystems Conference in Puerto Varas, Chile on September 30 and was presented with a medal in recognition of the event.
The founding director of the University of Rochester's Center for RNA Biology, Maquat has spent her career deciphering the many roles that RNA plays in sickness and in health. She's an international leader in the field and is credited with several major discoveries that are informing a new generation of therapies for a wide range of genetic disorders.
Her lecture, titled "Nonsense-mediated mRNA Decay in Human Health and Disease," described her discovery of nonsense-mediated mRNA decay or NMD and how this important surveillance system protects against mistakes in gene expression that lead to disease. She also discussed ongoing work on how misregulation of NMD in Fragile X Syndrome, the most common single gene cause of intellectual disability and autism, results in neuronal defects that typify this disorder.
Thank you to Dianne Edgar, MD and Terry Platt, PhD for their generous gifts to the Center over the years
Thursday, October 3, 2019
Thank you, from the Center of RNA Biology members, to Dianne Edgar, MD and Terry Platt, PhD for their generous gifts to the Center over the years.
Dr. Edgar is a Clinical Professor in the Department of Obstetrics and Gynecology at the URMC, and a Pediatrician at Parkwest Women's Health. Dr. Platt is a Professor Emeritus of the Department of Biochemistry and Biophysics at the URMC.
The Center will use the gift toward an RNA-centric seminar in their names, a testament to their generosity. Thank you, Dr. Edgar and Dr. Platt!
Training for a Cure: CF Researcher Raises Funds for EE
Friday, August 23, 2019
Emily's Entourage (EE) is incredibly honored to have a group of scientists dedicated to accelerating research and drug development for nonsense mutations of Cystic Fibrosis (CF). In the next year, 90% of the CF population could see the benefits of a life-saving drug on the market (currently pending FDA review and approval), but those in the final 10%, including those with nonsense mutations, will not benefit from these new breakthrough drugs.
And in fact, the commitment of the scientists run so deep that it is not merely limited to their "day job" at the lab advancing critical research. Rather, at times, it permeates many aspects of their lives. There is no better example of that than University of Rochester's John Lueck, PhD, who has decided not only to do research to speed breakthroughs and a cure for the final 10%, but also to raise the funds that will drive the critical research.
Over the last few months, Dr. Lueck has trained to climb The Matterhorn in the Swiss Alps, a mountain he tackled on August 22 that reaches almost 15,000 feet high. As a two-time EE grantee through the Catalyst for a Cure Campaign, he flipped the script and committed to raise funds for EE throughout the entire training process!
A Hidden Promise in the Language Cells Use to Communicate
Tuesday, May 8, 2018
Scientists have begun to implicate extracellular vesicles and exosomes in everything from cancer to viral infections to basic neural functioning. To Lynne Maquat, the J. Lowell Orbison Distinguished Service Alumni Professor in the Department of Biochemistry and Biophysics, the process shows how parts of the genome we used to think of as junk actually have important functions. “You could say that the host domesticated a viral sequence for its own purposes,” Maquat says. “That’s the beauty of our complexity—[these elements] allow tinkering or fine-tuning of genes.”
Maquat Wins the 2018 FASEB Excellence in Science Award
Sunday, April 22, 2018
Looking to RNA for Answers
Friday, March 23, 2018
Cancer is a group of diseases in which the body's cells divide uncontrollably and invade nearby tissues. Scientists at Wilmot Cancer Institute and the University of Rochester's Center for RNA Biology are working to understand more about how and why this happens.
One piece of the puzzle they're studying is ribonucleic acid, or RNA, which is found in all cells. RNA is made in the nucleus of a cell from our DNA, which holds the instruction manual for life. RNA puts those instructions into action.
RNA comes in many forms. One form, called messenger RNA (mRNA), carries those instructions out of the nucleus to the ribosomes, where proteins are made. These proteins are essential for functions ranging from digestion to protecting us from disease. If the mRNA has a bad copy of instructions, then either a faulty protein or no protein is created, leading to diseases like cancer.
Understanding the quality-control system
Lynne E. Maquat, Ph.D., director of the Center for RNA Biology, studies a quality control process that blocks cells from making faulty proteins. Called nonsense-mediated mRNA decay (NMD), this process comes into play when mRNA has a set of instructions with a mistake that will lead to short or incomplete proteins. NMD acts like a set of factory inspectors that find and destroy this mRNA before the faulty proteins can be made.
Sometimes, though, NMD doesn't catch the mistakes and harmful proteins are made. This process plays a part in one-third of all inherited diseases, such as cystic fibrosis and muscular dystrophy, and one-third of all acquired diseases, including a number of cancers.
One reason is that tumors can influence how these inspectors work. Maquat and her team are looking for ways to stop tumors from interfering with NMD with the goal of finding new ways to treat cancer.
Slowing cancer's growth
A team from Maquat's lab has also identified a protein called Tudor-SN that is important as cells prepare to divide. This protein controls many microRNAs, molecules that are very small RNAs that control the expression of tens of thousands of genes.
The scientists found that when Tudor-SN is removed from human cells, levels of hundreds of microRNAs go up, putting the brakes on genes that encourage cell growth. This slows down the process of cell division known as the cell cycle, which goes awry in cancer.
Maquat and Reyad A. Elbarbary, Ph.D., a former post-doctoral fellow in Maquat's lab, have filed a patent application for methods that target Tudor-SN for the treatment and prevention of cancer. They continue to study how Tudor-SN works in concert with other molecules and proteins so that scientists can identify the most appropriate drugs to target it.
Other avenues
Scientists at the Center for RNA Biology are also looking at RNA's other roles in cancer to find new treatment strategies. For example, Mitchell O'Connell, Ph.D., is studying how microRNAs can interfere with the way genes are expressed and lead to cancer. He and his team are using the gene-editing technology known as CRISPR, which he has adapted to edit RNA, to learn more about the proteins involved in this process.
Yi-Tao Yu, Ph.D., and his team are studying various ways to modify mRNA so that it can override mistakes in genetic instructions. Sometimes there's a premature stop signal that orders a cell to stop reading the genetic instructions in mRNA partway through the process, resulting in an incomplete protein. The Yu lab is working to alter mRNA in ways that turn "stop" signals into "go" signals, creating full length proteins and preventing diseases like cancer.
The study of RNA biology is allowing scientists and physicians to explore entirely new treatment strategies for cancer and a wide range of other genetic and acquired disorders.
Learn more about the work being done at the Center for RNA Biology.
By Lydia Fernandez
Maquat Wins 17th Annual Wiley Prize in Biomedical Sciences
Tuesday, February 20, 2018
The Wiley Foundation today announced the 17th annual Wiley Prize in Biomedical Sciences will be awarded to Lynne E. Maquat for elucidating the mechanism of nonsense-mediated messenger RNA decay, a fundamental process whereby cells remove defective transcripts that can encode toxic proteins.
Dr. Maquat is a Professor at the Department of Biochemistry & Biophysics in the School of Medicine and Dentistry, the Founding Director of the Center for RNA Biology: From Genome to Therapeutics, the Founding Chair of the University of Rochester Graduate Women in Science, and the J. Lowell Orbison Endowed Chair at the University of Rochester in Rochester, New York.
"The 2018 Wiley Prize honors Dr. Maquat, whose work illuminated how our cells prevent production of toxic proteins by removing defective messenger RNAs," said Dr. Titia de Lange, chairperson of the awards jury for the Wiley Prize at the Rockefeller University in New York City.
"The Wiley Foundation honors research that champions novel approaches and challenges accepted thinking in the biomedical sciences. The work of the 2018 Wiley Prize recipient Lynne Maquat truly upholds this mission," said Deborah Wiley, Chair of the Wiley Foundation. "We are pleased to highlight the impact that her research on messenger RNA decay pathways has had in advancing our knowledge of the cellular cause of many human diseases."
Lynne Maquat Wins Vanderbilt Prize in Biomedical Science
Tuesday, November 28, 2017
Lynne E. Maquat, Ph.D., the J. Lowell Orbison Endowed Chair and Professor in the Department of Biochemistry and Biophysics at the University of Rochester School of Medicine and Dentistry is the recipient of the 2017 Vanderbilt Prize in Biomedical Science. Established by Vanderbilt University School of Medicine in 2006, the competitive prize honors women scientists with a "stellar record" of research accomplishments who have made significant contributions to mentoring other women in science.
Maquat is internationally known for a huge body of research that describes what goes wrong in our cells during disease. The founding director of the University of Rochester's Center for RNA Biology, she has spent her career deciphering the many roles that RNA plays in sickness and in health.
She's dedicated a great deal of time to helping women advance in scientific careers and in 2003 established the University of Rochester Graduate Women in Science program. Through monthly round-table discussions with high-profile speakers who are using advanced degrees in traditional and non-traditional ways, the program seeks to broaden awareness of what women can do with a Ph.D. Program members can apply for travel awards to attend conferences, seminars or other external events that will help them advance their careers.
Maquat, also a professor in the Wilmot Cancer Institute, is the recipient of other prestigious honors, including the Canada Gairdner International Award; the International RNA Society's Lifetime Achievement Awards in Service and in Science; the Federation of American Societies for Experimental Biology (FASEB) Excellence in Science Award; the Rochester ATHENA Award®; and election to the National Academy of Medicine, the National Academy of Sciences and the American Academy of Arts and Sciences.
Maquat is the 12th recipient of the Vanderbilt Prize in Biomedical Science and will receive the prize on November 29, 2018, when she is scheduled to give a Flexner Discovery Lecture. She will also meet with Vanderbilt faculty and mentor Vanderbilt Prize Scholars, women who are pursuing graduate studies in the biomedical sciences in the School of Medicine.
"We are thrilled that Dr. Maquat is being recognized for her pioneering work in RNA biology, which has catalyzed innovative areas of research and provided insight to the role of RNA regulation in human disease," said Jennifer Pietenpol, Ph.D., Vanderbilt University Medical Center Executive Vice President for Research and director of the Vanderbilt-Ingram Cancer Center. "She is a world-renowned scientist and an exceptional mentor, a role model for us all."
Lynne Maquat Delivers Harvey Society Lecture at Rockefeller University
Tuesday, November 7, 2017
Lynne E. Maquat, Ph.D., the J. Lowell Orbison Endowed Chair and Professor in the Department of Biochemistry and Biophysics and Director of the Center for RNA Biology gave a Harvey Society Lecture on October 19 at The Rockefeller University in New York City. The lecture series is one of the most prestigious in the country and the lecturers, who are selected by the Harvey Society's leadership council, are leading biomedical researchers from around the world.
Maquat discussed her career path and the discovery of nonsense-mediated mRNA decay (NMD), which she first described for humans in 1981. NMD is a cellular quality control mechanism that derails the production of unwanted proteins in the body that can disrupt normal processes and initiate disease. Maquat is known around the world for her work on NMD, which is critically important in both normal and disease states. UR President Joel Seligman and URMC CEO and SMD Dean Mark Taubman attended the lecture.
Founded in 1905, the Harvey Society sponsors seven lectures annually that are open to the public and are attended by hundreds of scientists from New York City and the surrounding areas. The Harvey Lectures are one of the great traditions in New York science and they are a place where scientists from different institutions meet regularly.
URMC Scientist Lynne Maquat Elected to National Academy of Medicine
Monday, October 16, 2017
Lynne E. Maquat, Ph.D., an internationally recognized researcher who studies what happens in our cells during disease, has been elected to the National Academy of Medicine. The honor is akin to an actor receiving an Emmy for an outstanding performance; current members of the Academy elected Maquat for her exceptional research in the field of RNA biology. The accolade places her amongst an elite group of scientists and physicians who have made significant contributions to health and medicine.
In studying RNA, a close cousin to DNA, Maquat has discovered intricate cellular processes that influence normal genes, as well as genes involved in a wide range of diseases. Her findings are leading to the development of new treatment approaches for everything from cancer and heart disease, to intellectual and developmental disabilities and other neurologic disorders.
“Lynne is at the forefront of a movement to transform our increasing understanding of RNA biology into promising therapies for virtually all disease processes,” said Mark B. Taubman, M.D., CEO of the University of Rochester Medical Center and Dean of the University of Rochester School of Medicine and Dentistry. “She is an incredible asset to the University and will undoubtedly bring a wealth of valuable experience and knowledge to the National Academy of Medicine.”
Formerly called the Institute of Medicine, the National Academy of Medicine is an independent organization of professionals from diverse fields that advises the nation and the international community on issues in health, medicine and related policy. For example, in 2016 the Academy released a report calling for more and better research into ovarian cancer, one of the deadliest cancers, and recently issued a report detailing how physicians can help combat America’s opioid crisis.
The J. Lowell Orbison Endowed Chair and Professor of Biochemistry & Biophysics and of Oncology at the University of Rochester School of Medicine and Dentistry, Maquat is also an elected member of the National Academy of Sciences and the American Academy of Sciences. She was the first individual from upstate New York to receive the prestigious Canada International Gairdner Award, the country’s top award for excellence in biomedical research, which she won in 2015.
The National Institutes of Health has continuously funded Maquat’s research for the past 34 years and she’s published more than 150 papers and reviews. She is the founding director of the University of Rochester Center for RNA Biology and founding chair of the Graduate Women in Science program. She’s committed countless hours to mentoring the next generation of researchers and advocating for young women in the sciences.
When she is formally inducted into the National Academy of Medicine in October 2018, Maquat will join four other active Rochester faculty members: Seymour I. Schwartz in the Department of Surgery: Elizabeth R. McAnarney in Pediatrics; Paul S. Frame in Family Medicine; and Robert C. Griggs in Neurology.
Protein identified in post-chemo cell death puzzle
Monday, September 11, 2017
Because anticancer drugs are designed to kill growing cells, they also affect normal, fast-growing cells—blood cells forming in the bone marrow, for example, and digestive, reproductive, and hair follicle cells. Chemotherapy may also affect cells in vital organs, such as the heart, kidney, bladder, lungs, and nervous system.
Researchers at the University of Rochester, collaborating with colleagues at MIT, Harvard, and the University of Oslo, have identified a protein that is required for cell death after undergoing chemotherapy—at least, it appears, in male mice.
“That was the unexpected part,” says Dragony Fu, an assistant professor of biology at Rochester and corresponding author of the study, regarding the sex-specific nature of the experimental results.
Maquat Honored with FASEB Award, Featured on People Behind the Science Podcast
Friday, August 25, 2017
Dr. Lynne Maquat, J. Lowell
Orbison Endowed Chair and Professor of Biochemistry and Biophysics in the School of Medicine and Dentistry, Director
of the Center for RNA Biology, and Chair of Graduate Women in Science at the University of Rochester was recently
featured on the podcast, People Behind the Science.
Lynne discusses her mentors and career milestones, and offers advice to junior scientists. Maquat recently received
the Federation of American Societies for Experimental Biology's Excellence in Science Award, which recognizes women
who have made outstanding contributions to science through research discoveries and by training the next generation
of scientists.
The entire interview can be listened to for free on
iTunes.
Payea and Mishra are Inaugural Recipients of the Sayeeda Zain Travel Award
Friday, July 14, 2017
The department of Biochemistry and Biophysics recently presented to the inaugural Sayeeda Zain Travel Award to Mathew Payea and Laxmi Narayan Mishra.
Matthew Payea is a 6th year graduate student in the Biochemistry Ph.D. program studying tRNA biology in laboratory of Eric Phizicky. Matthew received his Bachelors in Science from Eastern Illinois University, majoring in Biochemistry. Matthew used the funding provided by the Sayeeda Zain Travel Award to attend the 22nd annual meeting of the RNA Society in Prague, Czech Republic this past June. There, he gave a talk on his research defining an RNA decay pathway in yeast that destroys mutant tRNAs.
Laxmi Narayan Mishra is a postdoctoral associate working in Dr. Jeffrey J Hayes' Lab in the Department of Biochemistry and Biophysics, University of Rochester Medical Center. He has a Masters degree from University of North Bengal, Darjeeling, India and a Ph.D. in Biochemistry from Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India. His research is focused on how epigenetic modifications alter chromosome structure to facilitate gene expression. His Dr. Mishra will use the Sayeeda Zain Travel Award to attend and present his research findings at the international EMBO conference on "The Nucleosomes: From Atoms to Genomes" at Heidelberg, Germany, in August 2017.
The Sayeeda Zain Travel Award is given semi-annually to one or more graduate students and postdoctoral fellows in the Department of Biochemistry and Biophysics at the University of Rochester School of Medicine and Dentistry. The award honors the life and achievements of Professor Sayeed Zain, Ph.D., a longtime faculty member in the Department of Biochemistry and Biophysics. Learn more about the award and Dr. Zain.

Matthew Payea (left) with Dr. Jeffrey Hayes

Laxmi Narayan Mishra (left) with Dr. Jeffrey Hayes
Study: A New Way to Slow Cancer Cell Growth
Friday, May 26, 2017

Cells grow and divide during the cell cycle
Cancer is an extremely complex disease, but its definition is quite simple: the abnormal and uncontrollable growth of cells. Researchers from the University of Rochester’s Center for RNA Biology have identified a new way to potentially slow the fast-growing cells that characterize all types of cancer. The findings, reported today in the journal Science and funded by the National Institutes of Health, were made in kidney and cervical cancer cells in the laboratory and are a long way from being applied in people. But, they could be the basis of a treatment option in the future, the authors said.
Cancer: The Cell Cycle Gone Wrong
All cells go through the “cell cycle,” a series of events that culminate in orderly cell growth and division. In cancer, the cell cycle is out of whack; cells divide without stopping and invade surrounding tissues.

Lynne Maquat, Ph.D.
Researchers identified a protein called Tudor-SN that is important in the “preparatory” phase of the cell cycle – the period when the cell gets ready to divide. When scientists eliminated this protein from cells, using the gene editing technology CRISPR-Cas9, cells took longer to gear up for division. The loss of Tudor-SN slowed the cell cycle.
“We know that Tudor-SN is more abundant in cancer cells than healthy cells, and our study suggests that targeting this protein could inhibit fast-growing cancer cells,” said Reyad A. Elbarbary, Ph.D., lead study author and research assistant professor in the Center for RNA Biology and the department of Biochemistry and Biophysics at the University of Rochester School of Medicine and Dentistry.
Elbarbary, who works in the laboratory of senior study author Lynne E. Maquat, Ph.D., a world-renowned expert in RNA biology, adds that there are existing compounds that block Tudor-SN that could be good candidates for a possible therapy.
Putting the Brakes on Cell Growth
Maquat’s team discovered that Tudor-SN influences the cell cycle by controlling microRNAs, molecules that fine tune the expression of thousands of human genes.
When Tudor-SN is removed from human cells, the levels of dozens of microRNAs go up. Boosting the presence of microRNAs puts the brakes on genes that encourage cell growth. With these genes in the “off” position, the cell moves more slowly from the preparatory phase to the cell division phase.
“Because cancer cells have a faulty cell cycle, pursuing factors involved in the cell cycle is a promising avenue for cancer treatment,” noted Maquat, director of the Center for RNA Biology and the J. Lowell Orbison Endowed Chair and professor of Biochemistry and Biophysics.
Maquat, who also holds an appointment in the Wilmot Cancer Institute, and Elbarbary have filed a patent application for methods targeting Tudor-SN for the treatment and prevention of cancer. Research next steps include understanding how Tudor-SN works in concert with other molecules and proteins so that scientists can identify the most appropriate drugs to target it.
Keita Miyoshi, Ph.D., staff scientist in Maquat’s lab, served as lead study author with Elbarbary. Jason R. Myers and John M. Ashton, Ph.D. from the UR Genomics Research Center played an instrumental role in the study analysis.
Using rooster testes to learn how the body fights viruses
Thursday, April 27, 2017
Researchers from the University of Rochester Center for RNA Biology: From Genome to Therapeutics examined the role of piRNA in safeguarding the integrity of the genetic information in germ cells. It's known that piRNA -- a type of ribonucleic acid (RNA) that's found most readily in the testes and ovaries -- shields germ cells by silencing the genetic sequences of viral intruders. It's also known that defects or mutations in piRNA lead to infertility in humans and other animals. What's not known is how piRNAs are generated in the first place.
A team led by Xin Li, Ph.D., assistant professor in the departments of Biochemistry and Biophysics and Urology at the University of Rochester School of Medicine and Dentistry, analyzed rooster testes to find out.
Maquat Receives Lifetime Achievement Award in Science from International RNA Society
Tuesday, February 7, 2017

Lynne E. Maquat, Ph.D. has spent her career unraveling what happens in our cells during disease, making seminal contributions to our understanding of RNA's role in sickness and in health. She's also committed countless hours to mentoring the next generation of researchers and advocating for young women in the sciences. For these exceptional efforts, she's receiving the 2017 Lifetime Achievement Award in Science from the international RNA Society.
The J. Lowell Orbison Endowed Chair and Professor in the Department of Biochemistry and Biophysics at the University of Rochester School of Medicine and Dentistry, Maquat began her professional career studying inherited anemias. She discovered a quality control process that blocks the creation of toxic proteins that cause disease. Known as nonsense-mediated mRNA decay or NMD, this process plays a part in one third of all inherited diseases, such as cystic fibrosis and muscular dystrophy, and one third of all acquired diseases, including a number of cancers.
"This award recognizes Lynne's pioneering contributions to understanding the mechanisms of RNA, as well as her outstanding leadership, support and commitment to our field, including her role as a model for new generations of scientists," said Juan Valcarcel Juarez, current president of the RNA Society, who works at the Centre for Genomic Regulation in Barcelona, Spain.
James McSwiggen, CEO of the RNA Society, added, "I can't imagine a more appropriate choice of awardee."
Mitchell O'Connell Lab To Open
Monday, December 12, 2016
Current Postdoctoral Berkeley Fellow, Mitchell O'Connell, Ph.D. is set to open his new lab in April 2017, in the department of Biochemistry & Biophysics, at URMC. Currently Mitch is working in Jennifer Doudna's lab and his research aims to understand the mechanisms of RNA-mediated gene regulation through the development of new RNA-targeting tools based on CRISPR/Cas technology.
Welcome Mitch!
‘Antisense’ compounds offer new weapon against influenza A
Thursday, November 17, 2016
Challenging a long-held convention, University researchers have shown they can inhibit the influenza A virus by targeting its genomic RNA with “antisense” compounds.
Their findings, highlighted on the cover of Nucleic Acid Therapeutics, offer scientists a new way to attack an increasingly drug-resistant pathogen that causes an estimated 250,000 to 500,000 deaths a year.
“Antisense” compounds are synthesized with nucleotides, the building blocks of nucleic acid, often shown as various combinations of A, U, G and C. When the compounds – called antisense oligonucleotides (ASOs) – bind to the targeted genomic RNA, they block its ability to replicate.
The collaboration, involving the labs of Douglas Turner, professor of chemistry; Luis Martinez-Sobrido, associate professor of microbiology and immunology; and two researchers in Poland, reported that “antisense” compounds targeting one of the virus’ eight genomic RNA segments caused a five- to 25-fold reduction of influenza A virus in cell cultures.
“That’s a big difference,” Martinez-Sobrido says. “When mice are infected with 10,000 viruses, they all die. However, with 25 times less virus, all animals can survive infection and they don’t even develop symptoms.”
NIH Director Visits URMC, Says it’s an Exciting Time to be a Researcher
Monday, October 10, 2016
 Collins' first stop was lunch with 15 graduate students and postdocs who came prepared with a wide range of questions. The discussion covered the importance of communicating science to the public and policymakers, increasing diversity in biomedical research and new mechanisms to support young scientists at the start of their careers. Postdoctoral fellow Sarah Latchney and Ph.D. graduate student Solomon Abiola attended the lunch with Collins and describe the experience here.
Collins' first stop was lunch with 15 graduate students and postdocs who came prepared with a wide range of questions. The discussion covered the importance of communicating science to the public and policymakers, increasing diversity in biomedical research and new mechanisms to support young scientists at the start of their careers. Postdoctoral fellow Sarah Latchney and Ph.D. graduate student Solomon Abiola attended the lunch with Collins and describe the experience here.
Members of the Center for RNA Biology highlighted their most promising work for Collins and Center director Lynne E. Maquat, Ph.D., gave Collins a tour of her lab, where he met more trainees and junior researchers (admittedly, Collins' favorite part of visits like these).
 In his keynote address at the end of the day, Collins delivered an uplifting message to a packed house in the Class of '62 auditorium: it is an extremely exciting time to be in biomedical research, and after many lean years we are turning a corner, with the NIH budget finally increasing in real terms. He detailed several of the NIH's new programs, like the Human Microbiome Project, Big Data to Knowledge (BD2K), the Precision Medicine Initiative and the Cancer Moonshot.
In his keynote address at the end of the day, Collins delivered an uplifting message to a packed house in the Class of '62 auditorium: it is an extremely exciting time to be in biomedical research, and after many lean years we are turning a corner, with the NIH budget finally increasing in real terms. He detailed several of the NIH's new programs, like the Human Microbiome Project, Big Data to Knowledge (BD2K), the Precision Medicine Initiative and the Cancer Moonshot.
He applauded URMC on the renewal of the CTSI funding and cited the translational research conducted by Arthur J. Moss, M.D., which has led to new treatments for patients with Long QT syndrome (LQTS), and John J. Treanor, M.D., which is helping scientists in pursuit of a universal flu vaccine. Collins outlined several new funding initiatives, including the NIH Director's Early Independence Award, which is helping assistant professor Elaine L. Hill, Ph.D., study the impact of fracking on infant and child health.
Collins affirmed that the U.S. is the strongest biomedical research country in the world thanks to institutions like URMC. You can view his keynote, "Exceptional Opportunities in Biomedical Research," here.
American Health Council Names Dr. Harold Smith, Ph.D. to Education Board
Monday, September 19, 2016
Dr. Harold Smith, Professor at The University of Rochester, has been selected to join the Education Board at the American Health Council. Dr. Smith will be sharing his knowledge and expertise in the field of molecular biology, molecular virology, RNA biology, and drug discovery.
Dr. Harold Smith became involved in research after beginning his career as a professor in the Department of Biochemistry at The University of Rochester. As a biophysics professor, he utilized his knowledge and expertise in the areas of research and innovation of RNA, protein molecular biology, cell regulation, and drug discovery. Furthermore, Dr. Smith develops curriculum, teaches and mentors students from high school to postgraduate.
Dr. Harold Smith is also the Founder, President, and CEO of OyaGen, Inc. The objective of OyaGen, Inc. is to induce transient and beneficial changes in the protein expression and function in human tissues by involving the editing enzymes in targeting biomedically relevant pathways.
Dr. Harold Smith is a member of The American Heart Association, The Council on Atherosclerosis, The RNA Society, The American Society for Biochemistry and Molecular Biology and a fellow in the The Royal Society of Biology. In addition, Dr. Smith serves on the Scientific Advisor Board of Cannabis Sciences, Inc., IgxBio, Inc. and Trovita Health Sciences as well as the Editorial Board of the International Journal of Virology and AIDS, Frontiers in Microbiology, The Journal of Biological Chemistry, and The Journal of BioDiscovery.
Maquat Featured at Cornell-Ithaca Creativity Workshop
Saturday, July 30, 2016
J. Lowell Orbison Chair of Biochemistry and Biophysics, and of Oncology, Lynne Maquat, PhD, was a featured speaker at The Creativity Spark: a creativity workshop for scientists, a workshop put on by Cornell University, July 25.
The creativity workshop featured award winning scientists and scholars, including two Nobel Laureates, as they discussed the Creativity Spark and its role in science exploration.
URMC Team Revises Understanding of Genetic Code
Friday, July 1, 2016
Beth Grayhack, Ph.D., with lab
members and grad students
Christina Brule and Jiyu Wang
Scientists for years have known that the genetic code found in all living things contains many layers of complexity. But new research from the University of Rochester cracks the code more deeply, clarifying for example why some genes are inefficiently translated into proteins.
In a study published in the journal Cell, the researchers, co-led by Beth Grayhack, Ph.D., of the UR School of Medicine and Dentistry, discovered the existence and identity of 17 pairs of inefficient codons (DNA nucleotides or bases) within the genetic code.
Scientists have generally considered each piece of the genetic code (or codon) as a single “word” in a language. But the new data suggests some codon combinations act as compound words or phrases whose order and pairing has a significant impact on the translation of genes into proteins.
“Consider the words ‘pancake’ versus ‘cake pan,’ “ said Grayhack, an associate professor of Biochemistry and Biophysics, Pediatrics, and Cancer, in the Center for RNA Biology, at the UR Medical Center.
Review: Giving Gene Editing Technology CRISPR-Cas9 a Boost
Thursday, June 23, 2016
A new gene editing technology called CRISPR-Cas9 has taken the scientific world by storm. It allows researchers to quickly and easily make changes to the DNA of humans, animals and plants. The hope is that CRISPR-Cas9 may be used in the future to eliminate or correct faulty genes that cause disease.
In a recent issue of the journal Cell, Lynne E. Maquat, Ph.D. and Maximilian W. Popp, Ph.D. of the University of Rochester Center for RNA Biology describe how scientists can make this technology more efficient. Understanding the principles of nonsense-mediated mRNA decay (NMD), a cellular mechanism that Maquat discovered early in her career, will help anyone employing the technology achieve a better result -- namely, a more complete knock out or deletion of a desired gene.
Clara Kielkopf Receives EvansMDS Discovery Research Grant
Saturday, June 11, 2016
Biochemistry & Biophysics Associate Professor, Clara Kielkopf's project, entitled, Structural mechanisms and targeting of MOS-relevant pre-mRNA splicing factors has been selected by EvansMDS for funding for 2016. This year EvansMDS requested 12 full DRG proposals and were able to fund 6 of them. Their hope is that these findings will translate into improvements in therapy that can be delivered to MDS patients.
The Kielkopf lab investigates splicing defects in hematologic malignancies; roles of human pre-mRNA splicing factors in HIV-1 infectivity; development of engineered splicing factors for correction of splicing defects and splice sites and their associated proteins as therapeutic targets.
Harold Smith Publishes Commentary on RNA and DNA Editing
Sunday, June 5, 2016
Epigenetics is a popular, yet still mysterious concept in health and medicine. It’s the study of a variety of biological processes that alter the expression of our genes. Sometimes this involves modifying the structure of our chromosomes to mask or unmask genes, and other times the actual genetic code is changed in certain cells. Harold C. Smith, Ph.D., a longtime professor of Biochemistry and Biophysics at the University of Rochester School of Medicine and Dentistry has studied epigenetics in a research focus known as RNA and DNA editing since it was introduced two decades ago. He was invited to write a commentary on the progress and future of this research, published today in Trends in Biochemical Sciences, and answers a few questions about the subject.
Harold Smith Inducted into Royal Society of Biology
Friday, April 8, 2016
Dr. Harold Smith, Professor of Biochemistry & Biophysics has been inducted into the Royal Society of Biology.
A long time member of the department, Dr. Smith's primary interest is understanding the composition, regulation and structure of macromolecular complexes involved in regulating gene expression at the level of messenger RNA expression and processing. The lab's focus is on a platform of enzymes that change the genetic code at the DNA or RNA level by deaminating cytidine to form uridine. Current data suggest that this family of cytidine deaminase function with other proteins (auxiliary proteins) as holoenzymes complexes which we refer to as editosomes (for RNA) or mutasomes (for DNA). RNA editing or DNA mutational activity by these enzymes affect the protein coding capacity of mRNAs and thereby can diversify the proteins that are expressed by cells (the proteome). Please visit the Smith Lab for more information. Dr. Smith has a 30 year track record of teaching and mentoring graduate students, medical students and undergraduates at the University of Rochester and has lead curriculum design and reform for these programs.
The Royal Society of Biology (RSB), previously called the Society of Biology, is a learned society in the United Kingdom created to advance the interests of biology in academia, industry, education, and research. Formed in 2009 by the merger of the Biosciences Federation and the Institute of Biology, the society has around 16,000 individual members, and over 100 member organizations. In addition to engaging the public on matters related to the life sciences, the society seeks to develop the profession and to guide the development of related policies.
Professor Harold Smith to Organize Meeting on Drug Discovery
Thursday, March 31, 2016
The Clinical Science and Drug Discovery Conference had its inaugural meeting in 2015 in Baltimore, MD where Dr. Smith was asked to serve as a Keynote Speaker (and judge for poster sessions). The organizers of that meeting nominated him to organize this years meeting in Dundee, Scotland along with Drs. Ian Catchpole from GlaxoSmithKline in the UK and Nikolai Zhelev, professor at Abertay University, the hosting institution. The meeting will be held July 27-29. Dr. Smith will also deliver a keynote lecture at this meeting and chair a special topics session that he is bringing together on 'Host Cell Factors as Therapeutic Targets'. For more information, please visit the Drug Discovery Summit site, see also the CSDD Brochure.
B&B Professor Harold Smith and Oyagen's Drug Development Highlighted on Local TV for World Aids Day
Friday, December 4, 2015
OyaGen, a small medical research firm off Jefferson Road in Henrietta, has used federal grants for its HIV drug discovery programs with the goal of finding a cure. Dr. Harold Smith, Professor of Biochemistry & Biophysics at the University of Rochester, and the company's founder, president and CEO got his start as a molecular biologist studying heart disease.
"It became clear to me that the things we were doing to study heart disease and find out why things were happening translated directly into the HIV research arena," Smith said.
By 2010, things kicked into high gear. Advanced robotics were added allowing scientists to work with advanced chemistries. They've now identified a weak point in the HIV virus that's never been exploited before. Vif is a viral defense HIV releases into cells it infects. It destroys the body's natural defense against infections. OyaGen discovered a way to defeat HIV by disabling Vif.
"If we can proceed along track, we will be looking at entering clinical trials within a completely different way of approaching the virus and the disease within three years," Smith said.
Gloria Culver to Be Installed as Dean
Tuesday, December 1, 2015
Former Biochemistry graduate student and current Chair of Biology, Dr. Gloria Culver, will be
formally installed as dean of the School of Arts & Sciences during an investiture ceremony at 4 p.m. today in the
Interfaith Chapel on the River Campus.
University Trustee Ani Gabrellian ’84 will provide opening remarks,
followed by words from Provost Peter Lennie, the Robert L. and Mary L. Sproull Dean of the Faculty of Arts, Sciences &
Engineering. Mariette Westermann, vice president of the Andrew W. Mellon Foundation, and Jon Lorsch, director of the
National Institute of General Medical Sciences, will serve as guest speakers. Following the ceremony, a reception will
be held in the Hawkins-Carlson Room of Rush Rhees Library.
Lynne Maquat Receives Canada’s Top Prize for Biomedical Research
Thursday, October 29, 2015

On October 29, Dr. Lynne E. Maquat received a 2015 Canada Gairdner International Award, for her work discovering and elucidating the mechanism of mRNA decay pathways. Dr. Maquat was accompanied during presentation of the award by University of Rochester President Joel Seligman, Dean Mark Taubman, and the US Ambassador to Canada, Bruce Heyman (see photo). The sold out annual black tie gala took place at the Royal Ontario Museum in Toronto, Canada and was attended by members of the health care, academic, private and public sectors. Among the attendees were Nobel Laureate Dr. Phillip Sharp, His Excellency the Right Honourable David Johnston, Governor General of Canada, and the Swiss and Japanese Ambassadors to Canada, who accompanied recipients of the Canada Gairdner International Award from those countries.
Leading up to this event, Dr. Maquat visited four local universities where she spoke to high school students about her personal story of how she became interested in research and what she hopes to achieve through her work. She also met with post-docs and graduate students at each university as well as speaking to faculty members about their research. Following the gala, Dr. Maquat attended and spoke at a 2015 Gairdner Symposium RNA and The New Genetics
at the University of Toronto, which she helped coordinate. Her last event occurred at the University of New Brunswick, Fredericton where she once again spoke to high school students On being a Scientist: Uncovering the mysteries of life
and met with post-docs and graduate students about their research. Dr. Maquat took every opportunity to be part of the National Program, where the goal of these programs is to contribute to Canadian science culture and innovation,
and to be part of the Student Outreach Programs where she helped realize one of the Gairdner Foundation's missions to inspire young people to consider a career in science, and to increase their awareness of the value of scientific research.
A local reception was also held to honor Dr. Maquat; pictures available here.
Maquat and Kurosaki Awarded Fellowship
Friday, April 24, 2015
Lynne Maquat, the J. Lowell Orbison Distinguished Service Alumni
Professor in the Department of Biochemistry and Biophysics, and Research
Assistant Professor, Tatsuaki Kurosaki, Ph.D. have been awarded FRAXA Postdoctoral Fellowship for their application
entitled, Re-purposing clinically approved drugs to dampen hyperactive nonsense-mediated mRNA decay in fragile X
syndrome
.
The FRAXA Research Foundation was extremely impressed with
their proposed research, and delighted to support this exciting work
. Funding of 45,000 has been authorized
for the period from May 1, 2015 to April 30, 2016.
Congrats to both Lynne and Tatsuaki!
Gloria Culver, Biochemistry Program Graduate, Appointed Dean of the School of Arts & Sciences
Wednesday, April 22, 2015
Gloria Culver, former Biochemistry graduate student in the Phizicky Lab, has been appointed dean of the School of Arts & Sciences, effective immediately. Culver is currently a professor of Biology and Biochemistry & Biophysics. Peter Lennie, the Robert L. and Mary L. Sproull Dean of the Faculty of Arts, Sciences & Engineering, made the announcement following a yearlong national search. Culver has been serving as interim dean since July 1, 2014.
Harold Smith Awarded Drug Development Pilot Award
Tuesday, April 14, 2015
Biochemistry & Biophysics Professor, Harold Smith, PhD has been awarded a Drug Development Pilot Award for his project, Development of an Assay for High Throughput Screening for Antagonists of the Ebola VP40 Protein Function
. The project was externally reviewed by leading drug development researchers and received a meritorious score. To learn about Dr. Smith's research please visit the Smith lab site.
Congrats Harold!
Blocking Cellular Quality Control Mechanism Gives Cancer Chemotherapy a Boost
Friday, March 27, 2015
A University of Rochester team found a way to make chemotherapy more effective, by stopping a cellular quality-control mechanism, according to a study published today in Nature Communications.
The mechanism is known as NMD (nonsense-mediated mRNA decay), and scientists found that exposing breast cancer cells to a molecule that inhibits NMD prior to treatment with doxorubicin, a drug used to treat leukemia, breast, bone, lung and other cancers, hastens cell death.
The research team, led by Lynne E. Maquat, Ph.D., director of the Center for RNA Biology at the University of Rochester, acknowledges that the work is in the early stages and a long way from being applied in humans. But, they believe their data provide insights that could lead to new treatment strategies for cancer patients in the future.
Lynne Maquat Receives 2015 Gairdner International Award
Thursday, March 26, 2015
Lynne E. Maquat, Ph.D. received the 2015 Gairdner International Award for the discovery and mechanistic studies of nonsense-mediated mRNA decay, a cellular quality control mechanism that derails the production of unwanted proteins in the body that can disrupt normal processes and initiate disease. She is one of five scientists honored with the award, which is given every year to recognize the achievement of medical researchers whose work contributes significantly to improving the quality of human life.
The J. Lowell Orbison Endowed Chair and Professor of Biochemistry and Biophysics at the University of Rochester Medical Center, Maquat is known around the world for her work on nonsense-mediated mRNA decay, which is critically important in both normal and disease states. She is considered the uncontested pioneer on the subject and in 2011 was elected to the National Academy of Sciences for her exceptional research, which has been published in more than 130 peer-reviewed scientific articles.
Maquat is the first scientist from upstate New York to receive the Gairdner International Award, which is recognized for its rigorous peer-led selection process. A panel of active Canadian scientists reviews all nominations and passes their recommendations to a board of two dozen senior scientists from across Canada, the United States, Europe, Australia and Japan. After in-depth study and review, board members cast votes for the nominees whose achievements rise above all others in their field. According to the Gairdner Foundation, of the 313 winners to date, 82 have gone on to receive a Nobel Prize in Physiology or Medicine, a testament to the quality of the awardees.
The award was also highlighted in the Opinion pages of Saturday’s Democrat and Chronicle in the Thumbs up, thumbs down
section: Thumbs up: For Dr. Lynne Maquat, who is one of five biomedical researchers from around the world to win this year's Gairdner International Award. The University of Rochester Medical Center scientist has joined a prestigious group. Since 1959, more than a quarter of the Gairdner International winners have gone on to win a Nobel Prize, too.