URMC / Labs / Topham Lab / Projects / Computational modeling and tool development for understanding human immune responses to influenza
Computational modeling and tool development for understanding human immune responses to influenza
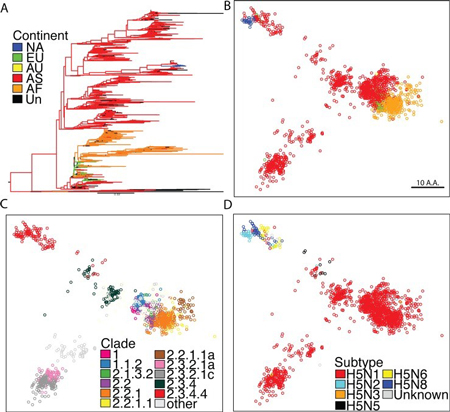
Comprehensive analysis of H5 virus Hemagglutinin. (A) Phylogenetic tree of influenza hemagglutinin proteins constructed from complete set of H5 HA sequences. Nodes were colored by continent of isolation. (B) SBM analysis on all publically available HA sequences for H5 viruses, sequences colored by continent of isolation. (C) Sequences colored by clade, only clades with >50 sequences were colored. (D) Sequences color by NA subtype.
Using publicly available sequence data from influenza viruses, as well as sequence data from our lab, we are developing mathematical models of how people respond to vaccination. The tools we have developed are useful for understanding the evolution of influenza viruses over time, for choosing optimal potential pandemic vaccine strains for stockpiling, for understanding how prior immunity to influenza affects vaccination, and simulation of alternative vaccination strategies that can achieve more broadly protective immune responses. Using a variation of a “shape-space” model originally developed by Derik Smith and Alan Perelson, we can simulate vaccination with different antigenic variants of influenza as well as the antibody responses that mirror the antigenicity of the viruses and vaccines. As the quality of the information we can gather on human immunity to flu, along with highly detailed information on antigenic properties of the viruses, we can consider the possibility of “personalized vaccination” in which the formulation of the vaccines could theoretically be tailored to an individual’s immune system. The idea is to fill gaps in immunity and achieve more broadly cross-reactive immune responses that might protect against many strains of flu.