Role of Proinflammatory Signaling in Glaucoma
The molecular signaling pathway leading from initial glaucomatous injury to retinal ganglion cell (RGC) death remains incompletely defined. Recent studies have supported a novel role for inflammatory extrinsic signaling as an early driver of glaucomatous neurodegeneration. Genomic analysis after ocular hypertension (OHT) revealed that astrocytes become reactive prior to RGC loss. However, when astrocyte reactivity was impaired, RGC death was exacerbated after both OHT and optic nerve crush (ONC) glaucoma-relevant injuries, suggesting activated astrocytes have a beneficial role in mitigating RGC degeneration. In addition to altered astrocytic reactivity, studies have suggested that proinflammatory cytokines are early drivers of RGC loss after OHT. For instance, cytokines known to be involved in neuronal injury are elevated in the retina in multiple animal models of glaucoma. A recent study showed genetic deficiency of three cytokines—complement component 1, subcomponent q (C1q); interleukin 1 alpha (Il1a); and tumor necrosis factor (Tnf)—lessened RGC death after axonal injury. Interestingly, this study suggested these molecules are expressed by microglia and primarily act on astrocytes, which in turn affect RGC viability. Despite the potential importance of these cytokines in driving RGC death after mechanical axonal injury, key questions about these molecules remain regarding their role in glaucoma relevant RGC death. In this project we are exploring the role of IL1A, TNF, and C1Q signaling as drivers of RGC death after axonal injury. This area of investigation is aimed at providing new insight into the role of extrinsic signaling in glaucomatous neurodegeneration and will determine whether manipulating C1Q, TNF, and/or IL1A signaling is a viable target for treating glaucoma.
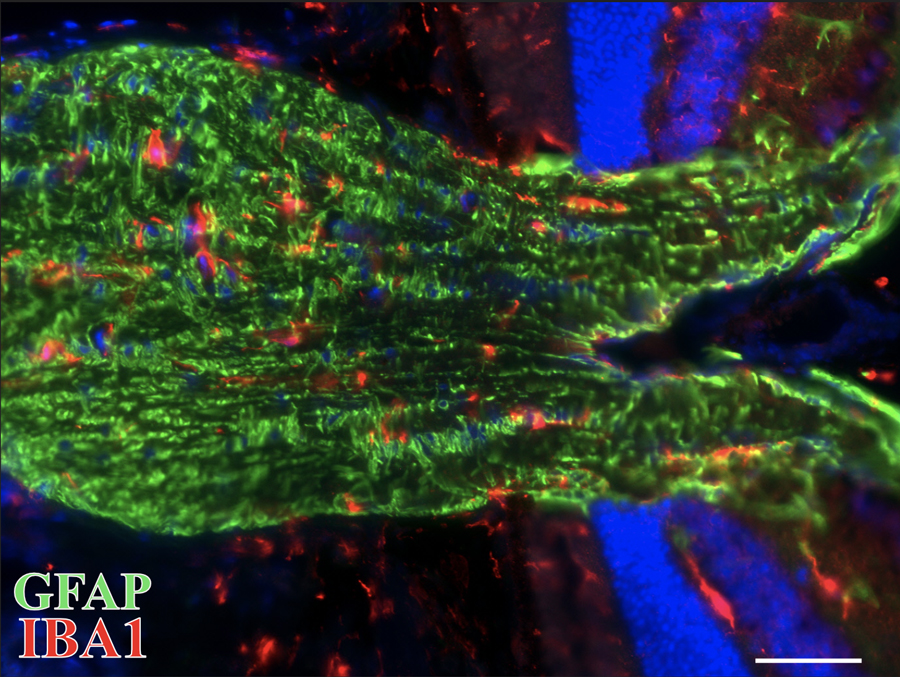
Optic Nerve Head (ONH) section labeling astrocytes (GFAP, green) and microglia (IBA1, red) under normal homeostatic conditions providing support to RGC axons.