Projects
Photoreceptor replacement therapy
Left: Andrea Campbell and Soumaya Bel Hadj, right: In vivo AOSLO images of tissue scaffold with fluorescent photoreceptor precursors.
To restore naturalistic vision with normal retinal processing it would be desirable to restore vision at the level of the photoreceptors themselves. This requires not only delivering photoreceptor precursor cells capable of maturing and forming synaptic connections into the sub-retinal space, but also that patient’s bipolar cell synaptic partners and downstream processing are functionally intact despite many years of photoreceptor loss.
In collaboration with David Gamm at the University of Wisconsin we have been tracking the survival and distribution of fluorescently tagged photoreceptor precursor transplants in the living eye.
Optogenetic therapy

Even after photoreceptors have degenerated the ganglion cells, the output neurons of the retina, often persist - can we make these cells light sensitive? This is the idea behind optogenetic vision restoration, where ‘optogenes’ cause light sensitive proteins to be expressed in cells that don’t normally have them and so these cells can be used as photoreceptors instead.
Adaptive optics calcium imaging allows us to evaluate and improve these vision restoration strategies in the living eye by recording activity in foveal retinal ganglion cells. Using the light gated channel ‘ChrimsonR’ we have demonstrated restored responses in foveal ganglion cells that have lost their photoreceptor input and shown that the expected patterns of activity are generated in response to patterned visual stimuli. We are currently investigating the longevity of optogenetic vision restoration in vivo and evaluating the potential impact of retinal remodeling after photoreceptor loss.
Understanding the Fovea
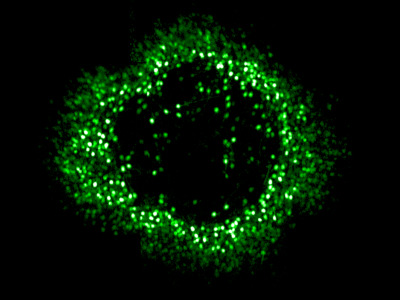
Ring of foveal ganglion cells expressing GCaMP in vivo.
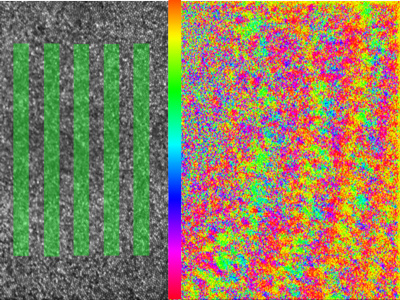
Phase response of retinal ganglion cells to a rolling grating stimulus presented at the fovea.
Despite its importance, relatively little is known about the physiology of the fovea because its delicate structure makes it challenging to study electrophysiologically. At Rochester we have been using our unique adaptive optics calcium imaging platform to investigate the fovea in vivo.
By performing calcium imaging while also using the AO system to present high resolution, precisely stabilized visual stimuli, we have been able to map the location of hundreds of retinal ganglion cell (RGC) receptive fields at the fovea. The spatial arrangement of the cone photoreceptors is well preserved in the spatial arrangement of RGCs, despite the dramatic developmental changes which generate the specialized foveal structure. This orderly mapping is of relevance to vision restoration therapies that aim to directly stimulate the retinal ganglion cells themselves. We are also beginning to classify the retinal ganglion cell types found in the fovea using coloured stimuli.
I have a longstanding interest in the physiological optics of the fovea. Extended photoreceptor axons ‘Henle Fibres’ radiate from the foveal cones to their displaced bipolar cells and interact with macular pigment molecules to produce a radially symmetric polarizing filter which mediates the human perception of the polarization state of light. This visual effect is known as ‘Haidinger’s brushes’. I’ve previously explored the threshold and rotational dynamics of the phenomenon using psychophysical testing and am interested in the optical interactions which give rise to it. I often use Haidinger’s brushes in outreach activities – almost everyone can discern the polarization state of light with the naked eye!
Biological Imaging
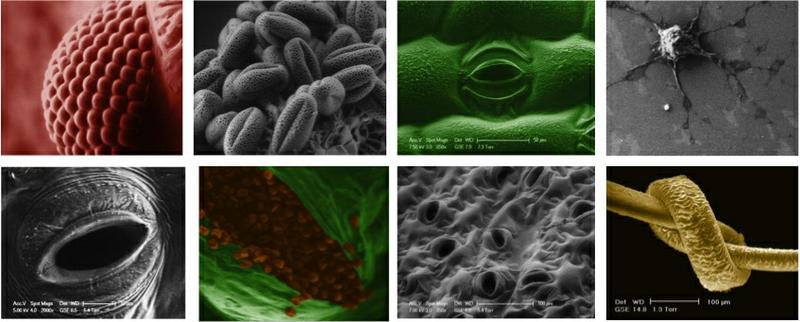
A selection of ESEM images from a compound eye, to a human hair.
As a PhD student at the Cavendish laboratory at Cambridge University I explored potential applications of Variable Pressure or 'Environmental' Scanning Electron Microscopy as a method for imaging dynamic biological processes. I did this work under the supervision of Prof. Dame Athene Donald FRS in the Biological and Soft Systems Sector of the Physics Department. In 2011 I was commissioned by the BBC to produce high definition images of the closure of stomatal pores using the new techniques developed during my PhD, for the BBC 2 documentary 'How to Grow a Planet'. If you are interested in learning more about VP ESEM I recommend taking a look at 'Principles and Practice of Variable Pressure: Environmental Scanning Electron Microscopy(VP-ESEM)' by Dr Debbie Stokes.