Regulation of Alveolar Formation
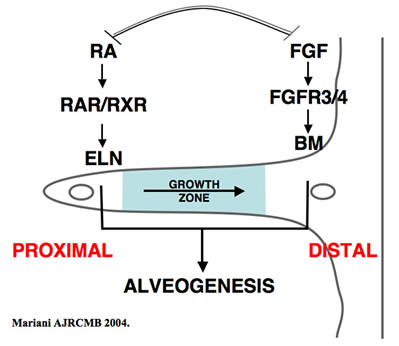
Alveolar elastic fibers are critical for respiratory structure and function. Elastic fibers are uniquely responsible for the property of passive tissue recoil, which is necessary for expiration and proper ventilation. Irreversible impairment of lung function in destructive lung diseases, such as emphysema, results specifically from the loss of elastic fibers. Likewise, numerous models of deficiency in developmental elastic fiber formation (elastogenesis) are associated with incomplete airspace formation. Paradoxically, some animal models of abnormal airspace formation, or chronic lung disease of the newborn, display excessive elastin accumulation associated with airspace pathology. In summary, these data indicate that correct spatial, temporal and quantitative alveolar elastogenesis is required for proper lung maturation and is necessary for maintenance of lung structure/function. Our long-term goal is to understand the factors that promote alveolar formation (alveogenesis) and maintenance, particularly those responsible for elastogenesis.
Alveolar elastic fibers accumulate specifically at the tips of secondary crests, at the site of alveolar entrance rings. Tremendous heterogeneity in mesenchymal cell phenotype within the alveolar wall has been appreciated, including myofibroblasts, lipofibroblasts, pericytes, endothelial cells and frank vascular smooth muscle cells. Although the importance of elastin in lung structure and function is generally appreciated, there remains a deficiency in our understanding of the ontogeny of alveolar elastogenic cells and the factors that regulate elastic fiber production during development. Data supports the concept that elastin expression is an inherent feature of a subpopulation or distinct lineage of mesenchymal cells. During our studies identifying the cellular and molecular mechanisms contributing to secondary crest elongation using the FGF receptor (FGFR)-3/FGFR4 compound deficient mouse, we identified IGF1 as a putative airspace elastogenic factor. We find that IGF1 promotes proliferation-dependent induction of elastin gene expression in isolated lung fibroblasts, and ex vivo cultures of lung tissue. Our data are consistent with the hypothesis that IGF1 promotes an elastogenic lineage of alveolar fibroblasts. This is particularly intriguing given the neonatal lethality, and long-appreciated, but poorly described lung pathology, associated with deficiency in IGF1 signaling. We hypothesize that IGF1 is required for airspace formation by promoting an elastogenic lineage in undifferentiated mesenchymal cells. We also identified SDF1/Cxcl12 expression in the developing lung, and observed that expression of this chemokine in alveolar epithelial cells correlates with the frequency of elastogenic cells. While SDF1 has previously been implicated in recruitment of circulating mesenchymal progenitor cells to the lung during injury/repair responses in the adult and neonatal lung, little data exists regarding the role of this mechanism during normal developmental processes. However, recent studies strongly suggest that some alveolar interstitial cells are derived from an extra-pulmonary source during normal lung development. We propose the innovative hypothesis that elastogenic cell progenitors are recruited to the lung during alveogenesis.