Molecular Interaction Services
Measure Kinetic Rate Constants and Affinities of Molecular Interactions
A state-of-the-art BIAcore T200 instrument is available to measure the on- and off-rates and affinities of biomolecular interactions.
- Samples ranging from 100 Da low molecular weight compounds to MegaDa complexes can be analyzed.
- On-rates ranging from 103 to 107 M-1s-1 and off-rates from 10-5 to 0.5 s-1 can be measured.
- Temperature control allows measurements to be made from 4 °C to 45 °C.
- Multiple samples can be loaded in 96- or 384-well plates.
- Samples can be recovered for mass spectrometry analysis.
- Automated programming allows experiments to run to 48 hours without user intervention.
The volume and concentration of a given sample depends on the affinity of the interaction for its partner. A suggested starting point is to conduct a 10-fold serial dilution series to achieve a sample concentration range between 10-5 to 10-10 M with each sample concentration in a volume of ˜100 µL. A method for immobilization of one binding partner on the sensor chip surface, and a buffer for regenerating the surface will need to be developed. Dr. Jenkins can assist with the experimental design and implementation on a fee-per-service basis. Dr. Jenkins must train and approve all new users prior to independent use of the instrument (please see T200 User Guidelines).
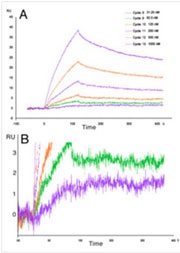
The image to the left consists of representative sensorgrams demonstrating very low RU changes detected by the BIAcore T200 for the small molecule (350 Da) binding to immobilized protein. (A) Concentration series. (B) Zoomed view of lowest RU changes (1 RU and 2.5 RU for 31 & 62 nM).
If a user has taken the BIAcore Basics training course (e.g. offered in Piscataway, NJ or San Diego, CA) only a brief introduction will be required prior to use of the local instrument. Likewise, if the user is experienced with other biomolecular interaction instruments, only 1-2 hours of preliminary instruction is anticipated. Otherwise, a training period of 1 day is required. Additional training in the software wizards available for data processing, or general consultation concerning experimental design and analysis, is offered on a fee-per-service basis. Alternatively, Dr. Jenkins can run and analyze experiments on an individual basis. Depending on the anticipated time-consumption of the experiment, it may be necessary to consult with the Executive Director about cost-sharing and the amount of time committed by the Staff Scientist to such projects on a 'fee-for-service' versus 'key personnel' basis.
Affinities and Thermodynamic Basis of Molecular Interactions
An isothermal titration calorimeter (ITC) for measuring the affinities and thermodynamics of molecular interactions is available in the laboratory of Dr. Kielkopf. Dr. Jenkins may use the ITC on behalf of facility users on a fee-per-service basis. A full characterization of the enthalpy, entropy, and free energy changes of the interaction can be obtained and the amount of material needed depends on the affinity of the interaction. A suggested starting point is 350 µLs of 10 µM solution of one component, and 80 µL of a 100 µM solution of the binding partner, although lower quantities may be used successfully depending on the system.
Fluorescent Monitoring of Molecular Interactions
The Department of Biochemistry has a Fluoromax-4 fluorimeter available for experiments that monitor affinities or dynamics of molecules by intrinsic tryptophan fluorescence or covalent attachment of a fluorophore. Although the exact amount of sample required will depend on the fluorophore chosen and the goal of the experiment, a starting point is a 20 nM sample in a volume of ˜500 µL(1 µg/mL of a 50 kDa molecular weight (MW) protein). For users that lack experience with Fluoromax instruments, Dr. Jenkins will be available to assist with experimental design and operation of the instrument on a fee-per-service basis.