Del Monte Institute for Neuroscience Speaker Series
Del Monte Institute for Neuroscience Speaker Series
The Measure of Change: Addressing the need for precise cognitive tools in the digital age
Vicroria Leavitt, PhD - Associate Professor of Neuropsychology, Columbia Univ. Irving Medical Center
Jan 22, 2025 @ 11:30 a.m.
Accurate measurement of cognitive change is essential for research and clinical care in neurologic populations. Precision tools allow the identification of neural substrates and permit the detection of meaningful change on the individual patient level. The digital age presents an opportunity to develop superior measurement instruments leveraging scalability, reliability, and ecological validity. We aimed to develop a tool that would be acceptable across cultures, in diverse socioeconomic, technology‐level, literacy‐level, and geographic populations. To optimize our tool to detect subtle cognitive change (e.g., as a marker of preclinical dementia), we created a 'cognitive stress test,' that would be impervious to floor and ceiling effects, and personalizable: the Language & Memory Test (LMT), a brief (4 minutes), multimodal, unsupervised digital test. To date, the LMT has been administered to over 1000 people in 4 countries in 3 countries, in urban and rural dwelling areas, to technology‐naive and low‐education individuals, and in ethnically/racially diverse communities. Initial psychometric properties of the test are encouraging; feasibility, acceptability, and usability are strong. Ongoing research with the LMT includes pilots in a USbased audiology clinic, in rural India, an MS clinic in Prague, and a collaboration with the Department of Biomedical Engineering at Columbia University to evaluate simultaneous EEG during LMT performance. We have discovered posture/behavior syllable‐related ensembles in the brain.
Medical Center | 2-6424 SMD Large Aud.
Host: Univ. Rochester SMD, Dept. Neuroscience, and Del Monte Institute for Neuroscience
Epigenetics: a mediator of environmental risk for Parkinson’s disease
Alison Bernstein, Ph.D. - Assistant Professor, Rutgers University Ernest Mario School of Pharmacy Department of Pharmacology and Toxicology
Mar 26, 2025 @ 11:30 a.m.
While there is debate about the magnitude of the relative contributions of genetic and environmental risk factors in the etiology of sporadic Parkinsons disease (sPD), it is well-documented that environmental risk factors and gene-environment interactions play a critical role in disease pathogenesis in most PD cases. However, the relatively large contribution of environmental risk factors in the overwhelming majority of PD cases has been widely neglected in the field. Toxicant exposures that contribute to PD risk may be temporally separated from disease onset, increasing susceptibility of these neurons to aging and subsequent neurotoxic insults without causing dopaminergic dysfunction on their own. The epigenome, and DNA modifications in particular, are recognized as an important mediator or the long-term effects of exposures across the lifespan. Thus, my lab focuses on how epigenetic modifications mediate neurotoxicological effects and gene-environment interactions in PD. We recently identified epigenetic changes associated with PD in human post-mortem brain tissue in genes related to the PD risk gene LRRK2 and endolysosomal sorting (RAB32 and AGAP1), PARK7 (DJ-1), SLC17A6 (VGLUT2), PTPRN2 (IA-2β), and NR4A2 (NURR1), as well as genes involved in neuroinflammation, neurodevelopment, neurotransmitter packaging and release, and axon and neuron projection guidance. We have also developed a two-hit mouse model that combines developmental dieldrin exposure with the α-synuclein preformed fibril (α-syn PFF) model to model increased disease susceptibility. In this model, male mice developmentally exposed to dieldrin have increased susceptibility to α-syn PFF-induced neurotoxicity. We identified dieldrin-induced changes in DNA modifications from birth to 9 months in pathways related to neurodevelopment, dopaminergic neuron differentiation, synaptogenesis, synaptic plasticity, and glial-neuron interactions, suggesting that developmental dieldrin exposure disrupts epigenetic regulation of critical neurodevelopmental pathways, impacting the risk of late-life disease. This model offers an opportunity to explore the persistent effects of environmental exposures and identify pre-degenerative changes that occur before the onset of neurodegeneration, setting the stage for increased susceptibility to disease.
Medical Center | SMD Large Auditorium (2-6424)
Host: University of Rochester School of Medicine and Dentistry Departments of Neuroscience and the Del Monte Institute for Neuroscience
Neuroinflammation is a critical component and mechanisms of Chronic Traumatic Encephalopathy
Jonathan Cherry, PhD - Assistant Professor of Pathology and Laboratory Medicine, Boston University
Apr 30, 2025 @ 4:00 p.m.
Medical Center | K307 (3-6408)
Host: Department of Neuroscience and the Del Monte Institute for Neuroscience
Functional connectivity constrained simulations of visuomotor circuits in zebrafish
Eva Aimable Naumann, PhD - Assistant Professor of Neurobiology, Duke University
May 07, 2025 @ 11:30 a.m.
Medical Center | K207 (2-6408)
Host: Department of Neuroscience and the Del Monte Institute for Neuroscience
Network and cellular mechanisms linking sleep to memory processing
Sara Aton, Ph.D. - Professor of Molecular, Cellular, & Developmental Biology, Michigan Neuroscience Institute Affiliate, University of Michigan
May 21, 2025 @ 4:00 p.m.
A third of our adult lives, and more than half of our early development, is spent sleeping. The cognitive and mental health disruption caused by sleep loss is increasingly well appreciated. However, the field of neuroscience has only a rudimentary mechanistic understanding of how and why sleep states (i.e., non-rapid eye movement [NREM] or REM sleep), and disruption of these states, affect the brain’s function. This lecture will describe recent efforts to identify sleep-dependent cellular and microcircuit mechanisms that contribute to the consolidation of newly-formed memories. Our studies, using mouse genetic tools and simple, single-trial learning paradigms, suggest that sleep may facilitate memory storage through coordinated reactivation of "engram" neurons activated by learning, and recalibration of excitatory-inhibitory balance. Understanding these mechanisms may aid in modifying the progression of disorders like Alzheimer's dementia, where both sleep alteration and cognitive disruption are present.
Medical Center | Ryan Case Method Rm. (1-9576)
Host: Department of Neuroscience and the Del Monte Institute for Neuroscience
The role of NRXN1 in somatosensory processing: untangling a mixed phenotype
Ariel Lyons-Warren, MD, PhD - Assistant Professor of Child Neurology Research Navigator, Texas Children’s Hospital
Jul 29, 2025 @ 4:00 p.m.
Children with autism can exhibit complex sensory phenotypes including both hyper and hypo sensitivity. Treating these sensory differences is challenging due to lack of understanding of the underlying circuit mechanisms. Recent work has suggested that peripheral somatosensory changes can cause autism features in mice. We therefore sought to understand the central and peripheral contributions of a common autism gene, NRXN1, to somatosensory function. First, we generated mice lacking NRXN1 throughout all neurons (Nes-Cre;NRXN1flox/flox) and mice lacking NRXN1 exclusively in dorsal root ganglion neurons (Adv-Cre;NRXN1flox/flox). We assessed somatosensory sensitivity using Von Frey filaments, hot plate and cold plate. We also assessed somatosensory reactivity using tape on back. We compared responses between experimental animals and littermate controls using student’s t-test. Mice lacking NRXN1 throughout all neurons exhibited hyper-sensitivity to hot and cold temperatures and decreased responsivity to a piece of tape on their back. There was no difference in sensitivity as measured using Von Frey filaments. In contrast, mice lacking NRXN1 in dorsal root ganglion neurons exhibited only a decreased responsivity to a piece of tape on their back, with no differences to temperature. Therefore, we conclude that NRXN1 causes temperature hypersensitivity via a central mechanism while causing decreased responsivity via a peripheral mechanism. Central vs peripheral contributions to sensory processing from the same gene may explain complex sensory phenotypes in autism.
Ariel Lyons-Warren is an assistant professor of child neurology at Baylor College of Medicine. She earned her bachelor degree in neuroscience at Johns Hopkins University and then her MD/PhD at Washington University in St. Louis before completing a basic-neuroscience focused residency in child neurology at Texas Children's Hospital. Her research focuses on understanding the circuit mechanisms underlying sensory coding and the impact of disruption to these processes in children with neurodevelopmental disorders. Her work spans animal models using a variety of behavioral, electrophysiological, and cellular molecular techniques to clinical trials characterizing patterns of sensory differences. She is particularly interested in the difference between hyper and hypo sensitivity measured objectively in anima
Medical Center | K207 (2-6408)
Host: Department of Neuroscience and the Del Monte Institute for Neuroscience
“Traveling waves of neural activity shape computation across maps of visual space” - Del Monte Institute for Neuroscience Seminar
Lyle Muller, PhD - Assistant Professor Department of Mathematics, Western University Lab for Network Computation, Fields Institute
Sep 24, 2025 @ 10:00 a.m.
Large-scale recording technologies now enable simultaneous measurement of neural activity across cortical regions with high spatial and temporal resolution. Using new computational tech-niques we developed for these data, we found that visual stimuli evoke waves traveling outward from input sites in primary visual cortex (Muller et al., Nature Communications, 2014). This is a spatiotemporally structured pattern of neural activation, analogous to “the wave” in a stadium, but where only a few fans stand at each point. During controlled visual detection tasks, these neural traveling waves (nTWs) dynamically modulate both neural excitability and visual percep-tion (Davis*, Muller*, et al., Nature, 2020). nTWs thus actively affect visual processing — but what could be their computational role? We have developed a new recurrent neural network model where brief input sequences trigger wave patterns that forecast upcoming inputs (Benigno et al., Nature Communications, 2023). This theoretical model demonstrates nTWs could enable predictive spatiotemporal computation by embedding continuous structures that unfold across maps of sensory space. These findings lead to a general framework for computation with spatio-temporal dynamics in topographically organized recurrent neural networks (Budzinski*, Busch* et al., Communications Physics, 2024; Liboni*, Budzinski*, Busch* et al., PNAS, 2025).
Medical Center | Class of '62 Aud (G-9425)
Host: Krishnan Padmanabhan & Anna Kolstad
“The Neural Basis of Expert Sound Perception”
Melissa Caras, PhD - Department of Biology, Department of Hearing and Speech Sciences, University of Maryland
Sep 25, 2025 @ 1:00 p.m.
Medical Center | Adolph Lower Aud. (1-7619)
Host: The Hearing and Balance Research Collective
“Investigating the Lifelong Impact of Prenatal Polysubstance Exposure on Brain Development and Behavioral Vulnerability” - Del Monte Institute for Neuroscience Seminar Series
Siara Rouzer, PhD - Postdoctoral Research Fellow Department of Neuroscience & Experimental Therapeutics, Naresh K. Vashisht College of Medicine, Texas A&M University
Oct 01, 2025 @ 10:00 a.m.
Polysubstance use during pregnancy is a critical but understudied public health concern. Alcohol and cannabinoids, two of the most widely co-used substances among individuals of reproductive age, exert overlapping effects on neurodevelopment, yet their combined impact on offspring health remains poorly understood. My postdoctoral research leverages translational rodent models to investigate how prenatal alcohol and cannabinoid exposures, alone and in combination, alter cerebrovascular development and corticostriatal circuitry across the lifespan.
Using high-resolution in vivo ultrasound, we demonstrated that both alcohol and cannabinoid exposures independently reduced fetal cerebral blood flow, while co-exposure produced the most profound impairments, predicting increased perinatal mortality. To probe neural mechanisms underlying long-term outcomes, we applied transcriptomic and network-based approaches to the dorsomedial striatum, a hub for motor and reward processing. Weighted gene co-expression network analysis revealed that prenatal exposures not only shifted baseline expression of synaptic and extracellular matrix networks but fundamentally reprogrammed their relationships with behavior. Notably, co-exposure inverted or amplified gene–behavior associations in a sex-dependent manner, transforming protective transcriptional signatures into risk-promoting ones for alcohol seeking and hyperactivity.
Together, these findings establish that prenatal alcohol and cannabinoid exposures impair fetal vascular development and rewire corticostriatal networks that regulate motor and reward behaviors. This work underscores the importance of considering polysubstance use and biological sex when evaluating risk for fetal alcohol spectrum disorders and highlights novel molecular pathways for intervention.
Medical Center | K307 (3-6408)
Host: Ania Majewska & Tracy Preko
Mending the Mind: A Journey from Single Neurons to Prosthetics for Memory - Taubman Dean's Lecture
Itzhak Fried, MD, PhD - Professor & Co-Director, UCLA Seizure Disorder Center David Geffen School of Medicine
Oct 13, 2025 @ 3:30 p.m.

School of Medicine and Dentistry | Class of '62 Aud. (G-9425)
Host: George D. and Freida B. Abraham Foundation
From Sound to Meaning: Intracranial Recordings and the Neural Underpinnings of Speech
Liberty Hamilton, PhD - University of California, Berkeley
Oct 16, 2025 @ 1:00 p.m.
Medical Center | Adolph Aud. (1-7619)
Host: Hearing and Balance Research Collective
Seeds for Collaboration: Faculty & Postdoc Mixer Aimed to Foster Cross-Disciplinary Connections
Oct 21, 2025 @ 4:30 p.m.

School of Medicine and Dentistry | LeChase Assembly Rm. G-9576
“Neuroimmune and Metabolic Dysfunction in Alzheimer’s Disease and Addiction” - Del Monte Institute for Neuroscience Seminar Series
Leon Coleman, PhD - Assistant Professor Department of Pharmacology, UNC School of Medicine, Bowles Center for Alcohol Studies
Oct 22, 2025 @ 10:00 a.m.
Microglial-neuronal interactions impact neuronal circuit function and neuronal health with aging. Here, work identifying a role for microglia in alcohol use disorder and Alzheimer’s disease is presented. Further, an age-related disruption in neuronal lipid metabolism is implicated in the progression of Alzheimer’s disease, with proinflammatory microglia contributing to this phenomenon.
Medical Center | K307 (3-6804)
Host: Ania Majewska & Lia Calcines Rodriguez
“Narratives reverberate in the internal brain-body dynamic” - Del Monte Institute for Neuroscience Seminar
Lucas Parra, PhD - Harold Shames Professor of Biomedical Engineering, City College of New York (CCNY)
Nov 05, 2025 @ 10:00 a.m.

Medical Center | K307 (3-6408)
"Antiviral Defense and Metabolic Regulation of A Key NAD+ Metabolic Enzyme in Neurodegeneration" - Del Monte Institute for Neuroscience Seminar
Shu Feng, PhD - Staff Scientist, Beckman Research Center, City of Hope National Medical Center, Duarte, California
Nov 10, 2025 @ 12:00 p.m.
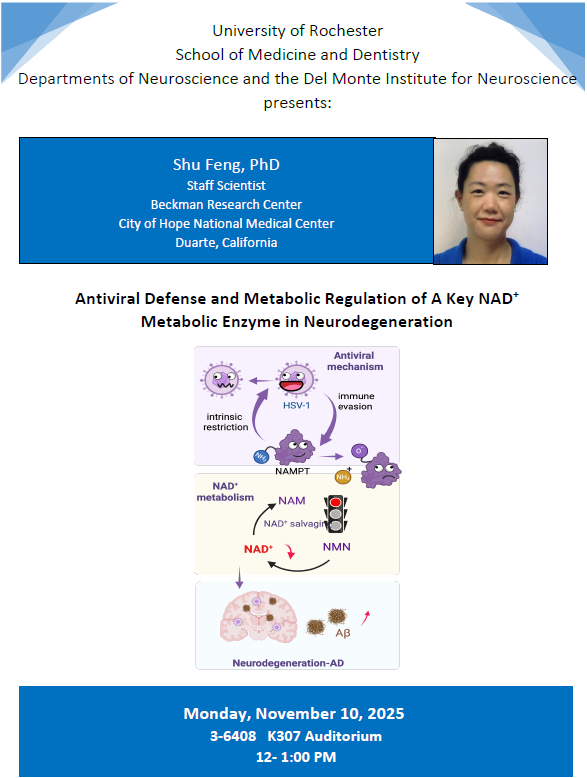
Medical Center | K307 (3-6408)
CANCELLED Mary Notter Lectureship - “A neural population journey from MT to the clinic”
Marlene Cohen, PhD - Professor of Neurobiology, The University of Chicago
Dec 03, 2025 @ 10:00 a.m.
Dr. Marlene Cohen is unable to visit on 12/3/25 so we will be rescheduling sometime later this winter or spring.

Medical Center | K307 (3-6408)
Host: Faculty Hosts: Ian Fiebelkorn and Manuel Gomez-Ramirez
Student Host: Alesandra Martin