Dopamine and Stress: Circuits Through the Extended Amygdala
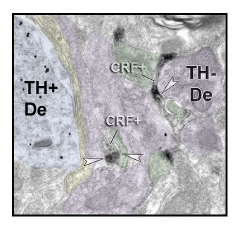
Figure 1. Pre-embedding dual-immunoreactivity electron microscopy (EM) shows DAB-filled CRF+ axons (gray) and Gold-labeled TH+ dendrites (black dots) in a lateral region of the A10 neurons. Excitatory synapses (asymmetric, black arrow heads), inhibitory synapses (symmetric, white arrow heads). Blue overlay= TH+ dendrite, purple overlay= dendrite, green overlay= dendritic spine, yellow overlay= presynaptic terminal
In 2000, we first focused on the potential impact of the extended amygdala on the dopamine system in primates (1, 2). Although the idea was novel—and somewhat controversial-- at that time, subsequent work from other laboratories and our own indicate that the extended amygdala-dopamine pathway is an important pathway mediating the effects of stress-induced behaviors, including depressive-like symptoms and drug-seeking.
We now focus on the microcircuitry of the extended amygdala-dopamine path, particularly the role of afferent inputs containing corticotropin releasing factor (CRF). CRF is abundant in the extended amygdala and is a co-transmitter that potently modulates the stress response. Even though dopamine release is best-studied in ‘reward’ paradigms, dopamine is strongly released during pain, novelty, and uncontrollable stress. Importantly, dopamine firing rates also increase during adolescence. Since many psychiatric disorders are first expressed in adolescence, dopamine regulation during this time would appear to be critical, and may be influenced by chronic stress.
Using a combination of tract tracing and conventional and electron microscopy, we are learning how information on aversive, or unexpected stimuli, processed by the extended amygdala can be channeled to specific dopamine output paths. We recently found that projections from the extended amygdala largely avoid the midline ‘classic’ VTA in primates, targeting instead the more lateral regions of the dorsal tier of dopamine neurons. This topography has implications for which DA-striatal paths are influenced by extended amygdala, or ‘stress-related’ circuits. We have further learned that CRF-containing terminals in these regions tend to form inhibitory synapses on GABAergic interneurons (rather than directly on the DA cells). This suggests a mechanism in which CRF-GABA terminals ‘inhibit the inhibitor’ and release the brakes on DA efflux under stressful circumstances. The individual differences in this system is being explored in adolescent and adult animals, and with respect to sex and social behaviors.
- Fudge JL, Haber SN (2000): The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience. 97:479-494
- Fudge JL, Haber SN (2001): Bed nucleus of the stria terminalis and extended amygdala inputs to dopamine subpopulations in primates. Neuroscience. 104:807-827