T Lymphocyte Migration: Live-cell Imaging by a Novel Fluorescence-based Technique
A number of fundamental physiological processes are dependent on cell surface integrins including embryogenesis, development, inflammation, immune responsiveness, wound healing, and regulation of cell growth and differentiation. The role of integrins in the immune system is particularly complex as these molecules regulate many aspects of the immune response. This is so because unlike cells within solid tissues, circulating leukocytes relocate during the course of immune reactions and in so doing dynamically adhere and de-adhere to cells of the vasculature and to other immune cells, as well to components of the extracellular matrix. A subset of integrins (LFA-1 (CD11a/CD18), VLA-4 (α4β1), and α4β7)) are expressed on T lymphocytes and play a major role in regulating recirculation and tissue specific homing.
The activity of integrins to bind ligands is dynamically and tightly regulated through conformational changes from inactive form to the active one that binds ligands with high affinity. Sustained and dysregulated integrin activation, resulting in abnormal T lymphocyte trafficking, and direct damage to the vasculature and the underlying tissue is known to contribute to pathologic conditions including vasculitis, atherosclerosis, stroke, rheumatoid arthritis, lupus, multiple sclerosis and Crohn’s disease. This makes lymphocyte integrin a promising therapeutic target. Therefore, to develop better-targeted and more effective anti-inflammatory therapies, it would be critical to understand how lymphocyte integrins and their ligands bind to one another and how leukocytes precisely regulate dynamic integrin activation during migration.
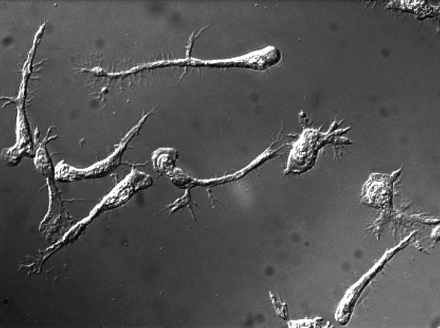
T cells elongate but are unable to detach their tails when the MyH9 myosin is blocked.
In order to visualize dynamic activation of lymphocyte integrin LFA-1 and its redistribution on the plasma membrane, we applied the quantitative imaging technique FRET (fluorescence resonance energy transfer) to living cells. Our current research focuses on integrin-mediated T lymphocyte polarization and migration. By employing advanced imaging techniques including two-photon microscopy and live cell FRET, we are trying to improve our understanding of the mechanisms of LFA-1 activation during chemokine mediated T lymphocyte migration.
References
- M. Kim, C. V. Carman, and T. A. Springer. 2003. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301(5640), 1720-1725.
- M. Kim, C. V. Carman, W. Yang, A. Salas, and T. A. Springer. 2004. The primacy of affinity over clustering in regulation of adhesiveness of the integrin αLβ2. J. Cell Biol. 167(6), 1241-1253.
- N.A. Morin, P.W. Oakes, Y.-M. Hyun, D. Lee, E.Y. Chin, M.R. King, T.A. Springer, M. Shimaoka, J.X. Tang, J.S. Reichner, and M. Kim. Non-muscle myosin heavy chain-IIA (My-H9) mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J. Ex. Med. 2008 Jan 21;205(1):195-205.