Research
Form, Function and Physiology
Ligand gated ion channels perform a remarkable feat. By coupling the energy of ligand binding to the opening of an electrical pore they are able to unleash a flood of ions, decoding chemical signals into appropriate electrical responses. The brain and the body employ a wide range of ligand gated ion channels which vary by endogenous activators, ion selectivity, response kinetics, cellular and sub-cellular distribution and a host a other parameters. In the MacLean lab we are interested in the workings of two such classes ligand gated ion channels: Acid Sensing Ion Channels (ASICs) and ionotropic Glutamate Receptors (iGluRs). How, precisely, does the structure of these channels give rise to their functional properties? To their kinetics, ligand and ion selectivites? And how does the brain employ those biophysical features to serve physiological ends? And finally, if we understand these processes well enough, can we design drugs to treat pathological signalling from these channels? To address these questions we combine patch clamp electrophysiology, fluorescence spectroscopy, kinetic and molecular modelling with standard molecular biology and biochemistry.
The Basics of ASICs
Acid Sensing Ion Channels (ASICs) are homo or heterotrimeric receptors which are activated by protons near the physiological range. Each subunit is roughly 500 amino acids with intracellular N and C termini, two transmembrane domains and a large extracellular domain which houses the proton sensors. In humans and rodents there are 4 ASIC genes which give rise to at least 6 different subunits. ASIC1a is widely expressed throughout the central and peripherial nervous system as well as other tissues. In many ways it is the ‘prototypical’ and best studied ASIC and the only one for which we presently have structural data. ASIC1b is a slightly lower affinity version of ASIC1a and is largely restricted to the peripheral nervous system. ASIC2a and b are also widely expressed and are the lowest affinity subunits, requiring pH’s around 4 or lower to maximally activate the channel. ASIC3 is the found mainly in the peripheral nervous system. It is the kinetically fastest of the bunch and has, perhaps, the most interest as a drug target given its role in pain sensation. ASIC4 does not respond to protons nor seemingly heteromerize with other ASICs. It’s function is unknown.
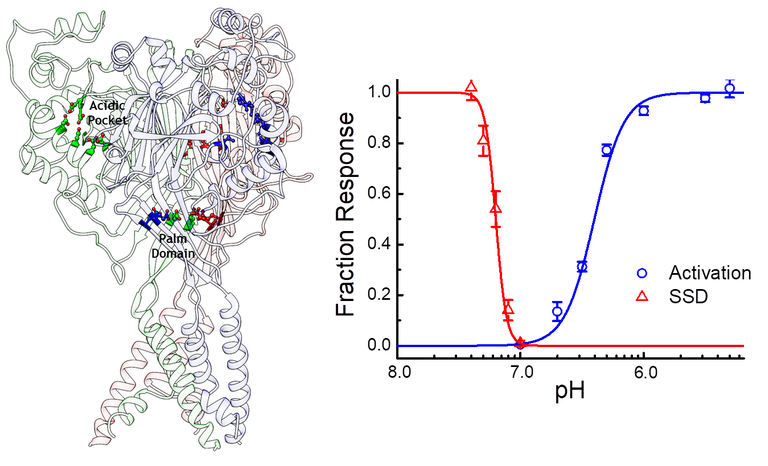
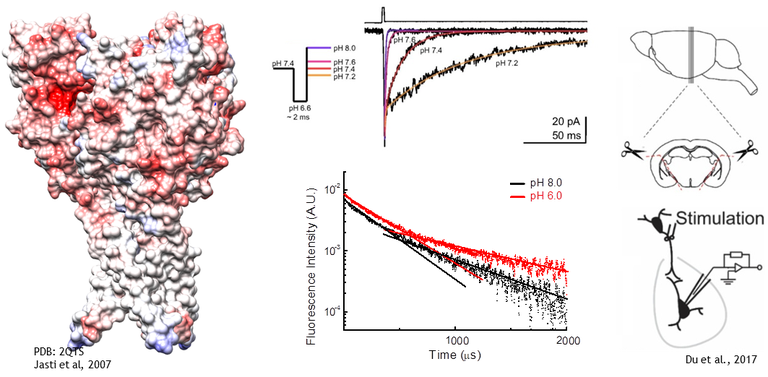
ASICs respond to protons in two general ways. Low concentrations of protons, around pH 7.4 or 40 nM, tend to inhibit ASICs without detectable activation in a process known as steady-state inactivation or desensitization (SSD). Higher concentrations, around 1 to 10 uM or pH 6 to 5, drive the channel to a Na+ selective open state. In the continued presence of protons ASICs undergo an additional conformational change, entering into a desensitized state. Protons activate ASICs via certain protonatable residues distributed in the extracellular domain. While we are still learning the mechanisms at play it seems that a cluster of acidic residues in the pocket the thumb and finger domains, as well as some in the palm domain, are especially important for driving activation and desensitization.
Ionotropic Glutamate Receptors
iGluRs are homo or heterotetramers which are activated by excitatory neurotransmitter L-glutamate. The general topology is the same throughout the family with a large extracellular amino terminal domain, a large extracellular ligand binding domain, three transmembrane domains along with a re-entrant pore loop and a variable length C-terminal tail. There are four classes of iGluRs with their own biophysical signatures and physiological roles. NMDA receptors, obligate heteromers composed of two GluN1 subunits and two GluN2A-D subunits, are relatively slow gating with the highest calcium permeability. These features, and their voltage dependent block by Mg2+ ions, enables them to play roles in the induction of synaptic plasticity and during development. AMPA receptors are built from GluA1-4 subunits. They are the fastest iGluR with extremely rapid activation, deactivation and recovery from desensitization, features which enable them to act as high fidelity relays between pre- and post-synapse. Kainate receptors are divided into the low affinity GluK1-3 subunits and the high affinity GluK4 and GluK5, the latter of which require the former to form functional channels. The physiological roles of KARs depend on their sub-cellular localization. Postsynaptic KARs contribute to exictatory synaptic transmission, presynaptic KARs are important for regulating the release probability of both glutamate and GABA and somatodendritic KARs regulate neuronal excitability through both iono- and metabotropic mechanisms. The delta GluD1 and GluD2 subunits complete the family. These are orphan receptors in that no endogenous activator has been found, though they are classed as iGluRs due to sequence and structural homology. Interestingly GluD2 serves a crucial role in the cerebellum where it acts as a trans-synaptic scaffold.
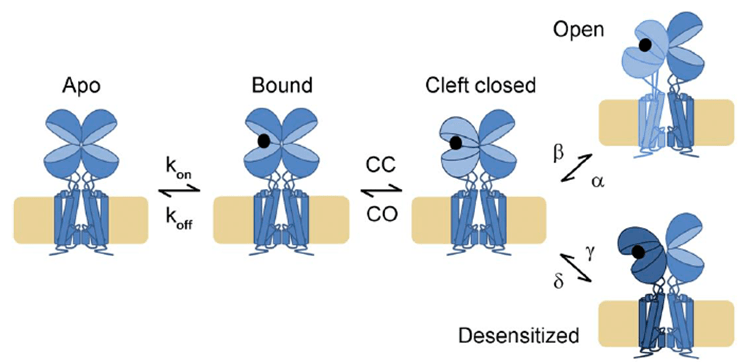
As with ASICs, iGluRs respond to glutamate by desensitization at low concentrations and activation/desensitization at higher concentrations. However, the structural basis for these processes is much better understood. iGluRs assembly as a dimer of dimers, with the ligand binding domains (LBD) braced back-to-back against their dimer partner. When glutamate binds it draws the LBD closed around it. This creates a high energy intermediate state, a stressed protein as the LBD wants to stay closed around glutamate while also maintaining close connection with its dimer partner and keeping the pore stably closed. To relieve this strain each subunit can ‘choose’ a reaction: either the lower lip of the LBD swings up in a move thought to twist open the pore or the upper lobe rocks down, breaking the interface between the LBDs and triggering desensitization. This framework is useful for AMPA/KA receptors at high agonist concentrations but the picture is less clear at lower occupancies and is greatly complicated by an array of gain and loss of function auxiliary proteins. NMDA desensitization also remains a bit of a mystery so there are many questions to be answered!
Funding
We are extremely happy to be funded by an R35 from NIH NIGMS (GM137591), the Paul Stark Endowment, a University Research Award and University of Rochester start-up funds.