Chromatin State Regulates Cellular Transitions
How cells transition from one state to another in vivo is a key question in biological sciences, with particular importance for the study of organismal development, carcinogenesis, and pluripotent reprogramming. In the early days of developmental biology, Conrad Waddington used the term ‘Epigenetics’ to refer to the process by which a cell transitions from one state to another during development, and how cells might be influenced by interactions between genes and their environment. Since this time, ‘Epigenetics’ has been redefined many times over, and now the definition includes molecular mechanisms for regulating gene activation or silencing (among others). Nonetheless, a complete understanding of how epigenetic mechanisms direct cell fate decisions is still lacking. My work is focused precisely on this issue.
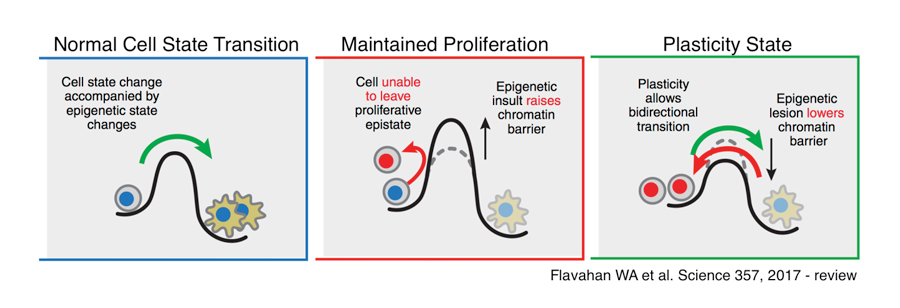
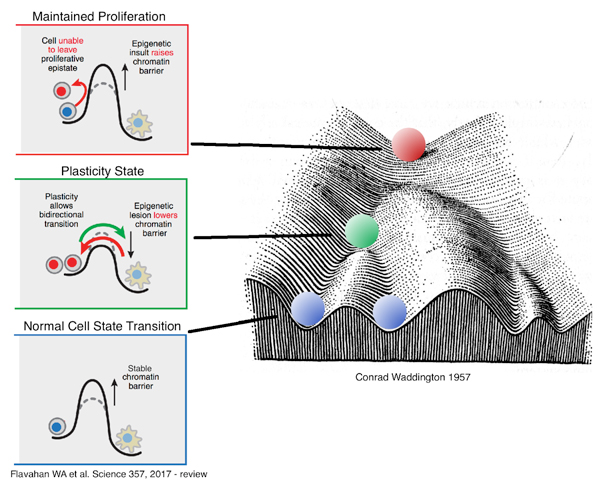
Leaders in the epigenetics field, including Dr. Brad Bernstein and Dr. Andy Feinberg, have developed elegant models to explain the role of epigenetic marks in cell state conversion, including during carcinogenesis. In Dr. Bernstein’s model, in order for a given cell to transition from one cell type to another, it must overcome an “energy wall.” In this model, epigenetic marks have the capacity to either raise or lower the “energy wall” and make it more difficult or easier for a given cell to transition between states. He refers to this low “energy wall” state as the “Plasticity” state. In this state, the “energy wall” is consistently low, and a cell might move more easily between states, and thus, contribute to carcinogenesis.
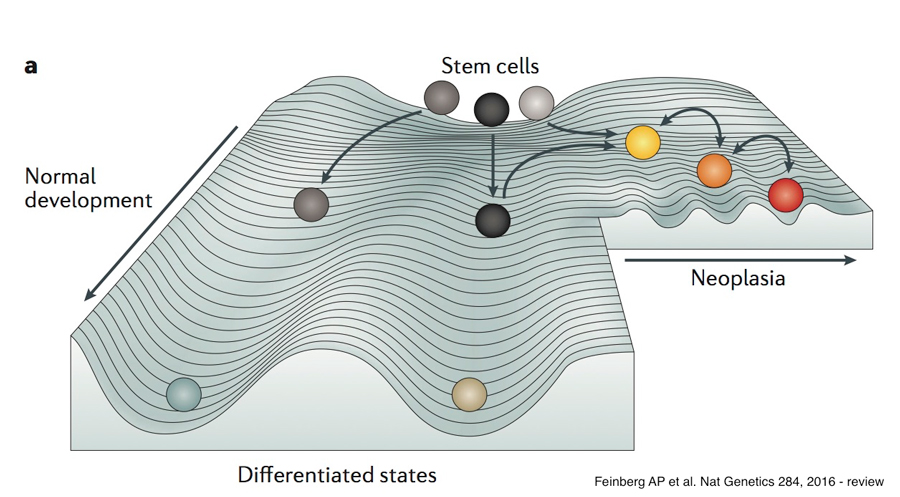
Dr. Feinberg’s model is quite similar to Dr. Bernstein’s, except it incorporates Waddington’s Epigenetic Landscape. Here, during normal development a given stem cell rolls downhill through the Landscape, and as it rolls it becomes confined within a particular cavernous valley (or lineage), where it is restricted, and eventually the cell comes to rest in a terminally differentiated state. In Dr. Feinberg’s model, a cell also has the potential to enter an alternative “Neoplasia” trajectory, where the valleys are much more shallow than the valleys of “Normal Development”, and therefore, the cell has a greater capacity to transition back and forth from one state to another. In this model, the depths of the valleys are similar to the “energy walls” from Dr. Bernstein’s model.
Plasticity Enable Cellular Transitions
We propose that cells enter a transition state, similar to Dr. Bernstein’s “Plasticity” state, during normal development, as they transition from one cell type to another. In the context of Waddington’s Landscape, this state might occur at the branch point between two valleys, immediately before a cell falls into one of two valleys and becomes committed to a particular lineage. We also hypothesize that the Placeholder nucleosome, which I previously characterized to enable DNA demethylation during germline-to-embryo transition, might function during developmental lineage transition points to lower “energy walls” and enable cell state transitions.
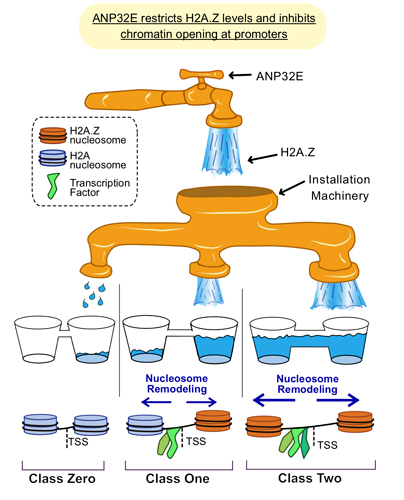
We have recently found that the key component of Placeholder nucleosomes, the histone variant H2A.Z is a universal chromatin accessibility factor functioning to promote transcription factor binding throughout the genome (Murphy KE et al. Nature Communications 2020). We also defined the histone chaperone ANP32E as a widespread inhibitor of chromatin accessibility. Loss of ANP32E causes the localization of H2A.Z nucleosomes to become more broad at promoters, leading to increase nucleosome remodeling and improved transcription factor binding. Our results also suggest that downregulation of ANP32E might enable cells to become more “Plastic”, enabling cells to exit defined states and transition to new states during developmental differentiation, regeneration, and carcinogenesis.