Chronic Pain
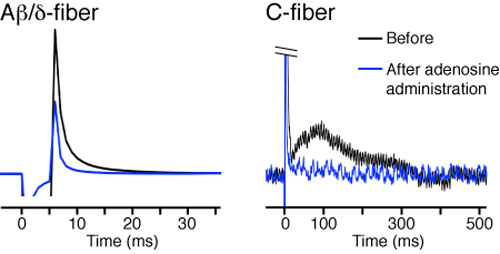
Adenosine reduces transmission of painful input through pain-specific nerve fibers.
Chronic pain disrupts the lives of over one and a half billion people around the world, upsetting their sleeping habits, limiting their functional capabilities and causing severe emotional trauma. Though there is no effective long-term solution, we currently treat chronic pain patients with medications such as opioids (morphine, codeine, etc.), non-steroidals (ibuprofen, asprin, etc.) and certain anti-depressants and anti-epileptics (amitriptyline, duloxetine, gabapentin, pregabalin, etc.), which attenuate the sensory perception of acute pain, typically by disrupting or dampening the conduction of pain signaling along non-specific pathways. However, none of the current medications effectively treat chronic pain and many are accompanied by unacceptable side-effects, such as slowed cognition, gastrointestinal complications, and in certain cases of prolonged use, a paradoxical hyper-algesic effect.
Part of the problem may be that current treatment paradigms are based on a fundamentally incomplete understanding of chronic pain’s neurobiological basis. Historically, the medical literature has been primarily dominated by a neuronally-driven model of chronic pain, whereby pain-specific neurons become hyper-excitable and prone to spontaneous and ectopic activity. But this model is incomplete -- what causes neurons to fire ‘spontaneously’, and what keeps them in an excitable state? Our lab investigates chronic pain from a totally different approach. We believe that broadening the pathological model to include glial-neuronal interactions will help answer those questions, and may well prove useful for developing cell-specific therapeutic targets.
We began our work by investigating the signaling interactions between astrocytes, and the pain-specific (alpha-delta and C-fiber) neurons of the spinothalmic tract. Astrocytes are prime suspects for chronic pain involvement, due to their widespread coupling via gap-junctions and close spatial proximity to neuronal synapses. In cell cultures, we demonstrated that astrocytes release ATP through unopposed connexin-43, a gap-junction protein typically coupled to other cells. Using bioluminscence in vivo imaging, we showed that wildtype, but not mice lacking connexin-43 released large amount of ATP in a spinal cord injury model of chronic pain. Consequently, mice with deletion of connexin-43 developed less severe chronic central neuropathic pain after spinal cord injury. ATP is an evolutionarily conserved signal transmitter. Bacterial lysis releases ATP, which acts as a danger signal to surrounding bacterium, signaling them to disperse. It is therefore not surprising that release of ATP has evolved to play a role in pain signaling, the danger alert system of more complex eukaryotes.
The consequences of ATP release are significant, given that many cell types (including neurons and microglia) express purinergic (ATP) receptors. Astrocytic release of ATP may constitute a direct, upstream, signaling pathway, by which astrocytes contribute to the development and maintenance of chronic pain. ATP binding to purinergic receptors on microglia is known to trigger the release of cytokines and other pro-inflammatory and pro-nociceptive signaling molecules. The identification of an astrocyte-pain-specific neuron signaling pathway fundamentally changes the pathological model of chronic pain, and may hold the key to developing targeted therapies capable of disrupting the vicious cycle of pathological pain signaling. Although efforts are just beginning, we have already shown that purinergic receptor antagonists, such as Brilliant Blue G, are capable of disrupting the purinergic signaling pathway.
Further Reading
Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M. Glia. 2012 Nov 0; 60(11):1660-70. Epub 2012 Aug 01.
Traditional acupuncture triggers a local increase in adenosine in human subjects. Takano T, Chen X, Luo F, Fujita T, Ren Z, Goldman N, Zhao Y, Markman JD, Nedergaard M. The journal of pain: official journal of the American Pain Society. 2012 13(12):1215-23.
Physiological and pathological functions of P2X7 receptor in the spinal cord. Cotrina ML, Nedergaard M. Purinergic signalling. 2009 Jun 0; 5(2):223-32. Epub 2009 Feb 11.
Deciphering migraine. Takano T, Nedergaard M. The Journal of clinical investigation. 2009 Jan 0; 119(1):16-9.
This project is supported by funding from NIH - National Institute on Drug Abuse. (Hemichannels, Astrocytic Release, and Neuropathic Pain).
Project Collaborators:
Dr. Ru-Rong Ji Harvard, Dr. John Markman, Dr. Takahiro Takano