The Specificity and Role of CD4 T Cells During the Response to Influenza Vaccines
The emergence of a pandemic influenza virus in Mexico in 2009, like other viruses with pandemic potential of earlier years has prompted great interest in understanding the nature of immunological memory to influenza, and whether and how previous encounters with influenza virus and vaccines influence our ability to mount a protective immune response when confronting relatively novel strains of this virus. One of the most significant gaps in our knowledge of protective immunity to influenza relates to that in the T cell compartment. Most studies evaluate serum antibody rather than the cellular responses to vaccination or natural infection.
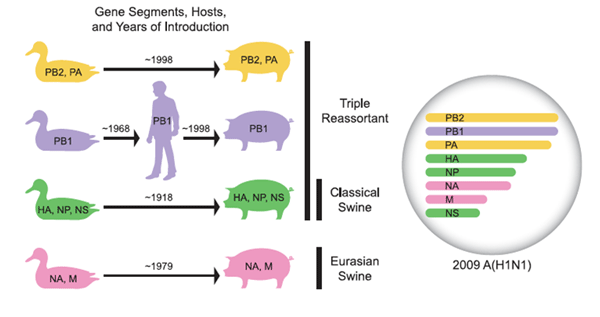
Genetic origin of pandemic strain of H1N1. (From Nancy Cox and colleague, 2009 Science.)
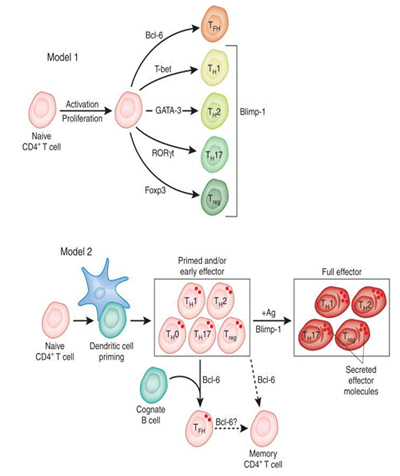
The production of high affinity, isotype switched antibodies in response to vaccines or pathogens depends on B cell interaction with antigen-specific CD4 T cells in the germinal centers of the peripheral lymphoid organs. It is now known that this activity is mediated by a specialized subset of CD4 T cells termed follicular helper T cells (Tfh) that are characterized by the expression of a subset of cell surface markers that promote the ability of the CD4 T cells to help B cells and to localize to the B cell follicles and participate in the germinal center reaction.
 Influenza-specific memory in humans is complicated by several unique characteristics of this pathogen. Humans typically encounter influenza for the first time through natural infection and then periodically, perhaps as frequently as every 3-4 years, and the immunological memory that is primed by natural encounters with influenza virus is then remodeled by vaccination or infection over an individual’s lifetime. Presumably, the influenza-specific CD4 T cell repertoire is re-shaped over time with subsequent encounters with alternative strains of influenza, with some specificities expanded and enriched in adulthood and others diminishing if they are never encountered again. However, although one can speculate on how these events affect anti-influenza memory, the forces that shape the influenza-specific T cell repertoire over a human lifetime are not known. For instance, we do not know whether efforts to focus the immune response to novel influenza strains with inactivated subunit vaccines enrich T cell specificity toward reactivity for the HA proteins at the expense of reactivity toward internal virion proteins, such as NP, or if frequent encounters with highly variable influenza viruses expands T cells specific for conserved proteins, leading to the ultimate dominance of these T cells in the memory compartment. Current experiments, involving multiparameter flow cytometry analyses of human peripheral blood cells isolated from vaccine recipients are designed to identify the key phenotypic characteristics of CD4 T cells circulating within human subjects that have the capacity to enhance or antagonize protective immunity to influenza vaccination.
Influenza-specific memory in humans is complicated by several unique characteristics of this pathogen. Humans typically encounter influenza for the first time through natural infection and then periodically, perhaps as frequently as every 3-4 years, and the immunological memory that is primed by natural encounters with influenza virus is then remodeled by vaccination or infection over an individual’s lifetime. Presumably, the influenza-specific CD4 T cell repertoire is re-shaped over time with subsequent encounters with alternative strains of influenza, with some specificities expanded and enriched in adulthood and others diminishing if they are never encountered again. However, although one can speculate on how these events affect anti-influenza memory, the forces that shape the influenza-specific T cell repertoire over a human lifetime are not known. For instance, we do not know whether efforts to focus the immune response to novel influenza strains with inactivated subunit vaccines enrich T cell specificity toward reactivity for the HA proteins at the expense of reactivity toward internal virion proteins, such as NP, or if frequent encounters with highly variable influenza viruses expands T cells specific for conserved proteins, leading to the ultimate dominance of these T cells in the memory compartment. Current experiments, involving multiparameter flow cytometry analyses of human peripheral blood cells isolated from vaccine recipients are designed to identify the key phenotypic characteristics of CD4 T cells circulating within human subjects that have the capacity to enhance or antagonize protective immunity to influenza vaccination.