Human B cell responses to influenza infection and vaccination: memory B cell studies
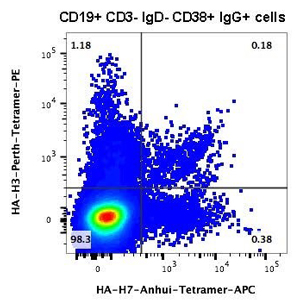
Flow cytometric analysis using tetramerized H3 and H7 HAs to identify HA-specific Ab-secreting cells. Cells in the upper right quadrant bind both the H3 and H7 probes, indicating specificity for the conserved HA stalk region.
The B cell response to influenza A virus infection or vaccination generates virus-specific antibodies (Abs) and memory B cells (MBCs) that play a key role in protective immunity. Preexisting influenza-specific Abs can block or quickly terminate infection in an immune individual. This is especially the case when the Abs bind to the viral hemagglutinin (HA), the viral attachment protein and the target of the most efficiently neutralizing Abs. If infection is established, MBC activation and differentiation into Ab-secreting cells is the basis for rapid and vigorous secondary Ab production that contributes to viral clearance. Importantly, preexisting HA-reactive MBCs are a critical requirement for strong neutralizing Ab responses to influenza vaccination.
Influenza-specific MBCs (and especially HA-specific MBCs) are a major research focus in our laboratory. In particular, we are interested in how the size and character of HA-specific MBC populations are modulated by exposure to the HA in different forms, for example, through infection versus vaccination. Our recent studies demonstrated that MBC populations expanded by influenza infection encompassed reactivity to a broad chronological range of HAs and also adapted to novel features of the HA of the infecting virus. This work relates closely to understanding the basis for the very broad HA-reactive Ab response to infection and preferential boosting of Abs against the HAs of viruses that caused significant early-life infection, a phenomenon known as “original antigenic sin”. We are also interested in how the form of HA exposure (infection versus vaccination; novel HA versus variant HA) influences Ab production against the HA head and stalk domains and how this relates to competition between head- and stalk-specific MBCs. Abs against the conserved HA stalk confer broad protection across influenza subtypes. Thus, understanding the processes that regulate stalk-specific Ab production is of major importance and relevant to the development of universal influenza vaccines.
Ongoing projects in the laboratory are investigating the dynamics of MBC populations specific for the influenza HA and other viral proteins in the context of human studies of (i) seasonal influenza vaccination, (ii) seasonal influenza infection, and (iii) vaccination with novel (e.g. avian) HAs. Other projects are investigating the relationship between birth-year and the breadth of reactivity of MBC populations. This measure could be a key predictor of the strength of protective Ab responses to novel influenza vaccines.