Projects
Project 1: Circuit interactions within the OCDnet, Haber
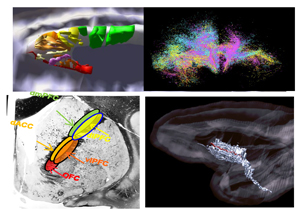 The pathophysiology of obsessive-compulsive disorder (OCD) is associated with circuit dysfunction in several cortical regions, (rostral anterior cingulate cortex (rACC), dorsal ACC (dACC), ventrolateral prefrontal cortex (vlPFC), orbitofrontal cortex (OFC), insula), and the rostral striatum, that, together integrate information related to behavioral inflexibility, resulting in persistent avoidance. These structures are referred as ‘nodes’ in the OCD network (OCDnet). Anatomically these nodes are interconnected extensively to mutually influence each other. Human imaging studies show that ‘hubs’, defined as regions with unusually high and diverse connectivity, exist within distributed networks. These hubs are considered important for integrating and distributing information. However, hubs are defined by imaging which cannot distinguish between inputs to a region that would integrate information, and outputs of a region that would distribute information. P1 combines quantitative anatomic methods with state-of-the-art imaging in both animals and humans to characterize the complexity of the interconnections of the OCDnet. Specifically, this project identifies hub locations within each OCDnet node and the location of their axons through major white matter bundles. Hubs are classified as integrative (i-hubs-regions that receive diverse inputs from multiple areas), distributive (d-hubs-regions that project widely), or both (central hubs-c-hubs-regions that receive and project to diverse areas). These data are used to explore how connectivity between nodes and hubs are involved in behavior and how disruption can lead to impaired behavioral flexibility in response to changing contextual cues in collaboration with P2-3 and for understanding how neuromodulation effects the circuits (P4-5).
The pathophysiology of obsessive-compulsive disorder (OCD) is associated with circuit dysfunction in several cortical regions, (rostral anterior cingulate cortex (rACC), dorsal ACC (dACC), ventrolateral prefrontal cortex (vlPFC), orbitofrontal cortex (OFC), insula), and the rostral striatum, that, together integrate information related to behavioral inflexibility, resulting in persistent avoidance. These structures are referred as ‘nodes’ in the OCD network (OCDnet). Anatomically these nodes are interconnected extensively to mutually influence each other. Human imaging studies show that ‘hubs’, defined as regions with unusually high and diverse connectivity, exist within distributed networks. These hubs are considered important for integrating and distributing information. However, hubs are defined by imaging which cannot distinguish between inputs to a region that would integrate information, and outputs of a region that would distribute information. P1 combines quantitative anatomic methods with state-of-the-art imaging in both animals and humans to characterize the complexity of the interconnections of the OCDnet. Specifically, this project identifies hub locations within each OCDnet node and the location of their axons through major white matter bundles. Hubs are classified as integrative (i-hubs-regions that receive diverse inputs from multiple areas), distributive (d-hubs-regions that project widely), or both (central hubs-c-hubs-regions that receive and project to diverse areas). These data are used to explore how connectivity between nodes and hubs are involved in behavior and how disruption can lead to impaired behavioral flexibility in response to changing contextual cues in collaboration with P2-3 and for understanding how neuromodulation effects the circuits (P4-5).
Project 2: Physiology and information processing of the OCD circuit, Monosov
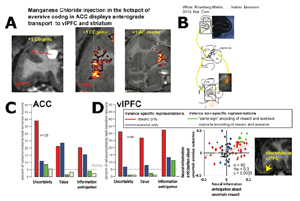 The control of adaptive behavior, at its most fundamental level, relies on appropriately weighting the rewards and aversive outcomes, and deciding when to take a risk to maximize reward, and when to play it safe. Malfunctions in these processes are associated with psychiatric disorders and maladaptive behavioral states, such OCD and anxiety. But despite their importance in everyday decision making and clinical settings, little is known about how aversive outcomes influence value- based decision making. In particular, in non-human primates, the closest animal model to humans, the neuronal mechanisms of how the brain evaluates and anticipates aversive events is poorly understood. P2 will (1) assess how the brain controls decision making under the risk and uncertainty of aversive outcomes in non- human primates and (2) in collaboration with other projects in the Conte Center, will utilize this information to shed light on the circuit mechanisms of, and novel therapies for, OCD and related pathologies. Aim 1 will utilize functionally targeted in-vivo anterograde tracing to identify regions within vlPFC, insula, and other regions that receive inputs from an area in ACC that has been functionally implicated in processing value and uncertainty of aversive outcomes. In the same animals, we will perform electrophysiological examinations of newly identified brain regions and assess whether and how they contribute to the processing of valuation, uncertainty, and receipt of aversive reinforcements. With P1, we will understand the relationship between functional properties of the OCD related brain areas and their anatomic connectivity. With P3-P5, we will relate single neuron data in non-human primates to ongoing therapy development and circuit mapping efforts in humans. Aim 2. will utilize a combination of computational and experimental methods to assess how the neural encoding of information about aversive reinforcements could give rise to OCD-like maladaptive behavior under aversive-outcome uncertainty. This Aim will utilize the probabilistic approach-avoidance task (PAAT), that we developed with P3, to determine the mental algorithms involved in aversive decision making under uncertainty and derive their neural underpinnings in the ACC-vlPFC-insula circuit. It will then disrupt the neural activity in identified sub regions of the circuit to obtain causal evidence for their contributions to specific aspects of behavioral control in PAAT. The unprecedented precision of single neuron recordings in this Aim will inform the interpretation of imaging data in other Projects. Furthermore, a wide range of direct manipulation methods for mediating OCD-related circuitry will be explored to facilitate the development of novel treatments for OCD in P5. In sum, these Center-integrated Aims represent crucial steps for our understanding of the neurobiology of OCD and more generally will broaden our understanding of the mechanisms of decision making. Particularly, in collaboration with other projects of the Center, they will shed light on how brain areas known to be crucially involved in OCD mediate our everyday decision making.
The control of adaptive behavior, at its most fundamental level, relies on appropriately weighting the rewards and aversive outcomes, and deciding when to take a risk to maximize reward, and when to play it safe. Malfunctions in these processes are associated with psychiatric disorders and maladaptive behavioral states, such OCD and anxiety. But despite their importance in everyday decision making and clinical settings, little is known about how aversive outcomes influence value- based decision making. In particular, in non-human primates, the closest animal model to humans, the neuronal mechanisms of how the brain evaluates and anticipates aversive events is poorly understood. P2 will (1) assess how the brain controls decision making under the risk and uncertainty of aversive outcomes in non- human primates and (2) in collaboration with other projects in the Conte Center, will utilize this information to shed light on the circuit mechanisms of, and novel therapies for, OCD and related pathologies. Aim 1 will utilize functionally targeted in-vivo anterograde tracing to identify regions within vlPFC, insula, and other regions that receive inputs from an area in ACC that has been functionally implicated in processing value and uncertainty of aversive outcomes. In the same animals, we will perform electrophysiological examinations of newly identified brain regions and assess whether and how they contribute to the processing of valuation, uncertainty, and receipt of aversive reinforcements. With P1, we will understand the relationship between functional properties of the OCD related brain areas and their anatomic connectivity. With P3-P5, we will relate single neuron data in non-human primates to ongoing therapy development and circuit mapping efforts in humans. Aim 2. will utilize a combination of computational and experimental methods to assess how the neural encoding of information about aversive reinforcements could give rise to OCD-like maladaptive behavior under aversive-outcome uncertainty. This Aim will utilize the probabilistic approach-avoidance task (PAAT), that we developed with P3, to determine the mental algorithms involved in aversive decision making under uncertainty and derive their neural underpinnings in the ACC-vlPFC-insula circuit. It will then disrupt the neural activity in identified sub regions of the circuit to obtain causal evidence for their contributions to specific aspects of behavioral control in PAAT. The unprecedented precision of single neuron recordings in this Aim will inform the interpretation of imaging data in other Projects. Furthermore, a wide range of direct manipulation methods for mediating OCD-related circuitry will be explored to facilitate the development of novel treatments for OCD in P5. In sum, these Center-integrated Aims represent crucial steps for our understanding of the neurobiology of OCD and more generally will broaden our understanding of the mechanisms of decision making. Particularly, in collaboration with other projects of the Center, they will shed light on how brain areas known to be crucially involved in OCD mediate our everyday decision making.
Project 3: Linking persistent avoidance with abnormalities in the OCDnet, Phillips
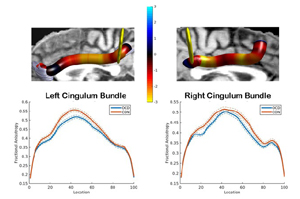 The goal of P3 is to characterize, in OCD, white matter (WM) bundle and functional neural abnormalities among regions in a putative OCD neural network that are associated with persistent avoidance, a characteristic feature of OCD. Our overarching Center renewal hypothesis, building on our present findings, is that persistent avoidance in OCD is a manifestation of dysfunctional connections among specific hubs, i.e., subregions that integrate and distribute information from multiple regions, in the ventrolateral prefrontal cortex (vlPFC) and rostral anterior cingulate cortex (rACC), and other regions in the OCD network, including the insula, dorsal ACC (dACC), orbitofrontal cortex (OFC) and rostral striatum. These dysfunctional connections lead to impaired behavioral flexibility in response to changing contextual cues, specifically in situations with uncertain aversive outcomes. In P3, we will recruit and examine 50 unmedicated/ serotonin reuptake inhibitor/clomipramine medicated participants with OCD, and 50 healthy participants (18-35 yrs; to minimize effects of long illness history and medication on neural measures). We will use state-of-the-art diffusion imaging (dMRI), using tractography, segmentation and tract profiling - tractometry - to examine WM bundles in the network. We will use functional Magnetic Resonance Imaging (fMRI) to examine activity, functional and effective connectivity (FC, EC) among network regions during a novel probabilistic approach avoidance task (PAAT) that we developed to examine the influence of uncertain rewarding and aversive outcomes on choice behavior, and neural activity, FC and EC during evaluation and anticipation of these outcomes. We will examine relationships among WM, activity, FC and EC abnormalities in the OCD network in OCD participants and the severity of OCD symptom dimensions associated with persistent avoidance, e.g., harm avoidance. Gender will be a covariate in analyses. Aim (A)1 will compare the microstructure of WM bundles that connect vlPFC, rACC, insula, dACC, OFC and rostral striatum in OCD vs. healthy participants, using a novel combination of tractography, segmentation and tractometry. A2 will compare activity within and FC and EC among these OCD network regions in OCD vs. healthy participants during the PAAT, to determine relationships among PAAT performance and fMRI abnormalities in OCD vs. healthy participants. A3 will examine relationships among OCD symptom dimensions that are relevant to persistent avoidance: e.g., harm avoidance, contamination/ washing, responsibility for harm/checking symptoms, and: WM and fMRI abnormalities in A1-2. P3 will benefit from expertise in: NHP neuroanatomy and physiology in P1, 2 (A2); dMRI data analysis in P1, Core B (A1); clinical assessment and treatment of OCD in P4, 5 (A3); the study of individual differences in neuroanatomy in Core C (A2-3); and integration of analyses across projects in Core D. In close collaboration with other projects and cores, P3 will be the first study to elucidate the specific WM bundle, and functional, OCD network abnormalities that are associated with persistent avoidance, to inform interventions for disorders characterized by this behavior.
The goal of P3 is to characterize, in OCD, white matter (WM) bundle and functional neural abnormalities among regions in a putative OCD neural network that are associated with persistent avoidance, a characteristic feature of OCD. Our overarching Center renewal hypothesis, building on our present findings, is that persistent avoidance in OCD is a manifestation of dysfunctional connections among specific hubs, i.e., subregions that integrate and distribute information from multiple regions, in the ventrolateral prefrontal cortex (vlPFC) and rostral anterior cingulate cortex (rACC), and other regions in the OCD network, including the insula, dorsal ACC (dACC), orbitofrontal cortex (OFC) and rostral striatum. These dysfunctional connections lead to impaired behavioral flexibility in response to changing contextual cues, specifically in situations with uncertain aversive outcomes. In P3, we will recruit and examine 50 unmedicated/ serotonin reuptake inhibitor/clomipramine medicated participants with OCD, and 50 healthy participants (18-35 yrs; to minimize effects of long illness history and medication on neural measures). We will use state-of-the-art diffusion imaging (dMRI), using tractography, segmentation and tract profiling - tractometry - to examine WM bundles in the network. We will use functional Magnetic Resonance Imaging (fMRI) to examine activity, functional and effective connectivity (FC, EC) among network regions during a novel probabilistic approach avoidance task (PAAT) that we developed to examine the influence of uncertain rewarding and aversive outcomes on choice behavior, and neural activity, FC and EC during evaluation and anticipation of these outcomes. We will examine relationships among WM, activity, FC and EC abnormalities in the OCD network in OCD participants and the severity of OCD symptom dimensions associated with persistent avoidance, e.g., harm avoidance. Gender will be a covariate in analyses. Aim (A)1 will compare the microstructure of WM bundles that connect vlPFC, rACC, insula, dACC, OFC and rostral striatum in OCD vs. healthy participants, using a novel combination of tractography, segmentation and tractometry. A2 will compare activity within and FC and EC among these OCD network regions in OCD vs. healthy participants during the PAAT, to determine relationships among PAAT performance and fMRI abnormalities in OCD vs. healthy participants. A3 will examine relationships among OCD symptom dimensions that are relevant to persistent avoidance: e.g., harm avoidance, contamination/ washing, responsibility for harm/checking symptoms, and: WM and fMRI abnormalities in A1-2. P3 will benefit from expertise in: NHP neuroanatomy and physiology in P1, 2 (A2); dMRI data analysis in P1, Core B (A1); clinical assessment and treatment of OCD in P4, 5 (A3); the study of individual differences in neuroanatomy in Core C (A2-3); and integration of analyses across projects in Core D. In close collaboration with other projects and cores, P3 will be the first study to elucidate the specific WM bundle, and functional, OCD network abnormalities that are associated with persistent avoidance, to inform interventions for disorders characterized by this behavior.
Project 4: Modulation of the OCDnet by conventional treatment, Baker, Brennan, Rauch
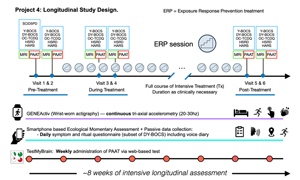 The main goal of the P4 is to study how conventional interventions for severe OCD – intensive exposure and response prevention (ERP) therapy and standard of care medication management – affect the putative OCDnet to further our understanding of how commonly available, efficacious treatments for OCD impact neural circuits involved in avoidance and aversive uncertainty. To achieve this goal, P4 will identify abnormalities in the vlPFC, rACC, insula, dACC, OFC and rostral striatum that track with performance on the PAAT and clinical symptom reports in individuals with OCD as they receive intensive treatment and undergo changes in illness burden over time. The project will follow 95 individuals with OCD using intensive longitudinal assessment of brain and behavior over the typical ~8wk treatment course. P4 will build on our group’s operational experience performing serial MRI (functional and structural) and detailed serial clinical assessments in OCD, and our toolkit for intensive longitudinal assessment, currently deployed in other NIMH-funded data collection efforts and developed with members of Core C, to identify changes in the OCDnet, persistent avoidance (as measured on PAAT and with exploratory device-based measures), and clinical changes in OCD. Three sets of comprehensive study visits (before, during, and after treatment) with MRI and clinical assessments will provide illness trajectory information for probing relationships between changing constructs at a coarse timescale, complemented by low-burden, digital phenotyping to capture variation at intermediate and finer time scales. Elucidating the ways in which the putative OCDnet changes in relation to changes in clinical and therapeutic parameters will provide targets for new interventions for OCD and help understand how and in which individuals currently available treatments achieve their optimal therapeutic benefit.
The main goal of the P4 is to study how conventional interventions for severe OCD – intensive exposure and response prevention (ERP) therapy and standard of care medication management – affect the putative OCDnet to further our understanding of how commonly available, efficacious treatments for OCD impact neural circuits involved in avoidance and aversive uncertainty. To achieve this goal, P4 will identify abnormalities in the vlPFC, rACC, insula, dACC, OFC and rostral striatum that track with performance on the PAAT and clinical symptom reports in individuals with OCD as they receive intensive treatment and undergo changes in illness burden over time. The project will follow 95 individuals with OCD using intensive longitudinal assessment of brain and behavior over the typical ~8wk treatment course. P4 will build on our group’s operational experience performing serial MRI (functional and structural) and detailed serial clinical assessments in OCD, and our toolkit for intensive longitudinal assessment, currently deployed in other NIMH-funded data collection efforts and developed with members of Core C, to identify changes in the OCDnet, persistent avoidance (as measured on PAAT and with exploratory device-based measures), and clinical changes in OCD. Three sets of comprehensive study visits (before, during, and after treatment) with MRI and clinical assessments will provide illness trajectory information for probing relationships between changing constructs at a coarse timescale, complemented by low-burden, digital phenotyping to capture variation at intermediate and finer time scales. Elucidating the ways in which the putative OCDnet changes in relation to changes in clinical and therapeutic parameters will provide targets for new interventions for OCD and help understand how and in which individuals currently available treatments achieve their optimal therapeutic benefit.
Project 5: Neuromodulation of OCD Circuitry, Rasmussen, Dougherty, Goodman
The overall goal of P5 is to study three direct circuit-based interventions for intractable OCD to advance our understanding of the neurocircuitry of the disorder and to (2) leverage that understanding in order to develop individualized approaches to treatment that will improve safety and efficacy. Our first aim is to examine the effects of MRI guided laser thermal ventral capsulotomy in twenty patients with intractable OCD using diffusion imaging to identify cortifugal axons affected by the lesions in individuals that are related to treatment response. We will also study the effects of lesions on individual resting state and task based (PAAT) functional connectivity and their relationship to treatment outcome measures. We will utilize novel acquisition and analytic algorithms for individuals designed by Cores B and C as well as interact with P1,3, & 4 to identify false negatives and positives in the diffusion scans and to compare imaging results in patients undergoing intensive non-surgical treatments to those with lesions. The second aim is to conduct a pilot feasibility study, using a double-blind crossover design, of a dual DBS implant in the vALIC and anterior cingulate bundle. The Medtronic Percept system will allow stimulation and recording of LFPs, in four patients with intractable OCD. We will stimulate and record in the vALIC and anterior cingulate, using pre-implant diffusion imaging and electric field modeling to identify which tracts are being activated. We will study the effects of stimulation at different contacts on the PAAT, and compare this to results obtained with electrophysiological recordings in the primate. Leveraging methodology developed by our collaborators (U Goodman, Dougherty) we will identify potential biomarkers of OCD provocation and treatment response. We will interact with P1 & 2 to compare anatomic and electrophysiological results across humans and primates as well as Cores B and C for imaging acquisition and analysis. Our third aim is to leverage ongoing studies of low intensity focused ultrasound in twenty-four patients with intractable OCD (Dougherty, Goodman PIs) and obtain pre post diffusion and task based (PAAT) functional imaging in those patients. We will compare these results to those found in lesions and DBS in the vALIC target and draw on CORE B and C as well as Project 1 and 2 to compare imaging results with nonsurgical OC subjects.
Core B: Structural Connections Core, Yendiki
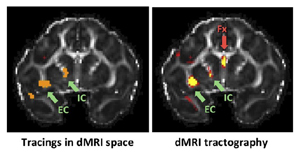 The goal of Core B is to provide support for mapping brain pathways relevant to the OCD network, in non-human primates and human subjects. This involves bridging between projects to translate information from anatomical tracing studies to neuroimaging studies that utilize diffusion MRI. The first aim of Core B is to collect diffusion MRI data with very high resolution and signal-to- noise ratio on post mortem macaque brains that have previously received tracer injections for P1; and to use these data to determine which of the pathways identified by diffusion tractography are accurate and which are artifacts due to the inherent limitations of diffusion MRI. The second aim of core B is to use the insights generated from the aforementioned macaque studies to guide the analysis of diffusion MRI data from human subjects in P3-5. Core B will provide these projects with optimized data processing pipelines for mapping the pathways likely to be involved in OCD, with the ultimate goal of investigating how these pathways differ between patients and healthy controls (P3), and how they are affected by noninvasive or invasive interventions (P4-5). Core B will advise the projects on all aspects of diffusion MRI data acquisition, quality assurance, and analyses to reconstruct and characterize bundles of the OCD network.
The goal of Core B is to provide support for mapping brain pathways relevant to the OCD network, in non-human primates and human subjects. This involves bridging between projects to translate information from anatomical tracing studies to neuroimaging studies that utilize diffusion MRI. The first aim of Core B is to collect diffusion MRI data with very high resolution and signal-to- noise ratio on post mortem macaque brains that have previously received tracer injections for P1; and to use these data to determine which of the pathways identified by diffusion tractography are accurate and which are artifacts due to the inherent limitations of diffusion MRI. The second aim of core B is to use the insights generated from the aforementioned macaque studies to guide the analysis of diffusion MRI data from human subjects in P3-5. Core B will provide these projects with optimized data processing pipelines for mapping the pathways likely to be involved in OCD, with the ultimate goal of investigating how these pathways differ between patients and healthy controls (P3), and how they are affected by noninvasive or invasive interventions (P4-5). Core B will advise the projects on all aspects of diffusion MRI data acquisition, quality assurance, and analyses to reconstruct and characterize bundles of the OCD network.
Core C: Precision Functional Neuroimaging Core, Wang, Lui
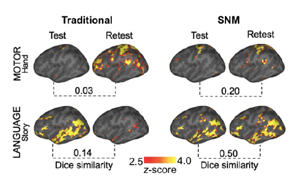 Functional neuroimaging experiments utilizing both resting state fMRI and task-based fMRI are proposed by individual projects across the Center, to accurately characterize the OCDnet. Given the substantial inter-individual variability of the OCDnet, these projects require advanced analytical approaches that can reliably and accurately map the OCD nodes in each participant. The central goal of the present core is to support these projects in acquiring and analyzing functional neuroimaging data using the cutting-edge techniques to achieve subject-level precision. Aim 1: The core will develop and implement individualized targeting strategies to identify homologous, subject-specific regions and networks based on resting-state fMRI. We will use a “maximizing between-subject homology” strategy and parcellate each subject’s cerebral cortex into very fine-grained functional clusters, each of which may consist of a single region or of several discrete regions. We will also identify homologous hub regions in humans based on connectivity patterns observed in non-human primates (NHPs). Aim 2: The core will develop and implement highly innovative denoising technologies that will dramatically improve the SNR of both task-based fMRI and resting state fMRI data. The denoising technology will be key to obtaining reliable task-induced functional responses not only at the individual subject level but also at the single trial level. Aim 3: The core will assist projects in continuing to implement the advanced functional MRI neuroimaging protocols developed during the previous funding period, that maximize within-individual signal properties and minimize anatomical distortion.
Functional neuroimaging experiments utilizing both resting state fMRI and task-based fMRI are proposed by individual projects across the Center, to accurately characterize the OCDnet. Given the substantial inter-individual variability of the OCDnet, these projects require advanced analytical approaches that can reliably and accurately map the OCD nodes in each participant. The central goal of the present core is to support these projects in acquiring and analyzing functional neuroimaging data using the cutting-edge techniques to achieve subject-level precision. Aim 1: The core will develop and implement individualized targeting strategies to identify homologous, subject-specific regions and networks based on resting-state fMRI. We will use a “maximizing between-subject homology” strategy and parcellate each subject’s cerebral cortex into very fine-grained functional clusters, each of which may consist of a single region or of several discrete regions. We will also identify homologous hub regions in humans based on connectivity patterns observed in non-human primates (NHPs). Aim 2: The core will develop and implement highly innovative denoising technologies that will dramatically improve the SNR of both task-based fMRI and resting state fMRI data. The denoising technology will be key to obtaining reliable task-induced functional responses not only at the individual subject level but also at the single trial level. Aim 3: The core will assist projects in continuing to implement the advanced functional MRI neuroimaging protocols developed during the previous funding period, that maximize within-individual signal properties and minimize anatomical distortion.
Core D: Neurocomputational Modeling Core, Frank, Shenhav
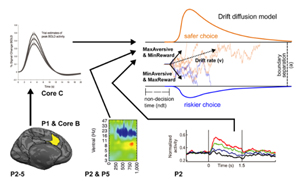 The overarching goal of the core is to provide a common formal framework that can incorporate measures of neural activity, connectivity, and behavior across Projects 1-5 to (a) quantify the functional roles of the OCDnet and its components in approach-avoidance decision-making and OCD symptomatology, and (b) predict changes in decision-making dynamics and symptom severity as a result of neural and clinical interventions. To achieve these goals, we will leverage (a) models of decision dynamics and their modulation by neural activity within individual circuit nodes, and (b) graph-theoretic models of interactions across circuit nodes. To quantify decision dynamics during the PAAT task, we will use hierarchical Bayesian parameter estimation of the drift diffusion model (HDDM), which enables reliable estimation of decision parameters and their modulation by trial-by-trial variance in neural signals, and supports Bayesian hypothesis testing for how these parameters may differ as a function of clinical status and neuromodulation. We have previously shown how such “computational biomarkers” can discriminate between patient conditions and symptoms better than traditional measures of behavior and brain activity, including in an approach-avoid context. We will test how PAAT choices are modulated by a combination of task variables (e.g., rewarding and aversive outcomes), neural activity across OCDnet nodes, and OCD symptom severity. Preliminary results show that the HDDM captures expected differences in choice dynamics (e.g., choice bias) between patients and healthy controls. To quantify task-related functional interactions across this circuit, we will use ancestral graph models, which measure the strength and direction of information flow across graph nodes. We will use this combination of modeling approaches to test for changes in decision and circuit dynamics resulting from targeted interventions (e.g., lesions, stimulation, treatment). Machine learning methods will quantify the degree to which such quantitative model fitting improves (1) classification of patient condition and (2) our ability to map changes in behavior, circuit dynamics, and disease course following interventions. Building on our extensive experience in neural networks and levels of computation involved in motivated learning and decision making across species, our computational framework will facilitate not only enhanced sensitivity to discriminate between clinical conditions, but will also identify hypotheses about the likely mechanisms involved, which will be tested via causal manipulations using the same quantitative framework. Contribution to Overall Center Goals & Interactions with Other Center Components. Our modeling framework will be applied to data across all Projects, including measures of connectivity (P1), behavioral and neural activity (P2-5), clinical measures (P3- 5), and influences of neural (P2&5) and behavioral (P4) interventions. Cores B & C will help with localization and estimation of neural activity. We will benefit from interactions amongst experts with complementary expertise in systems and cognitive neuroscience, psychiatry, engineering, and computational modeling.
The overarching goal of the core is to provide a common formal framework that can incorporate measures of neural activity, connectivity, and behavior across Projects 1-5 to (a) quantify the functional roles of the OCDnet and its components in approach-avoidance decision-making and OCD symptomatology, and (b) predict changes in decision-making dynamics and symptom severity as a result of neural and clinical interventions. To achieve these goals, we will leverage (a) models of decision dynamics and their modulation by neural activity within individual circuit nodes, and (b) graph-theoretic models of interactions across circuit nodes. To quantify decision dynamics during the PAAT task, we will use hierarchical Bayesian parameter estimation of the drift diffusion model (HDDM), which enables reliable estimation of decision parameters and their modulation by trial-by-trial variance in neural signals, and supports Bayesian hypothesis testing for how these parameters may differ as a function of clinical status and neuromodulation. We have previously shown how such “computational biomarkers” can discriminate between patient conditions and symptoms better than traditional measures of behavior and brain activity, including in an approach-avoid context. We will test how PAAT choices are modulated by a combination of task variables (e.g., rewarding and aversive outcomes), neural activity across OCDnet nodes, and OCD symptom severity. Preliminary results show that the HDDM captures expected differences in choice dynamics (e.g., choice bias) between patients and healthy controls. To quantify task-related functional interactions across this circuit, we will use ancestral graph models, which measure the strength and direction of information flow across graph nodes. We will use this combination of modeling approaches to test for changes in decision and circuit dynamics resulting from targeted interventions (e.g., lesions, stimulation, treatment). Machine learning methods will quantify the degree to which such quantitative model fitting improves (1) classification of patient condition and (2) our ability to map changes in behavior, circuit dynamics, and disease course following interventions. Building on our extensive experience in neural networks and levels of computation involved in motivated learning and decision making across species, our computational framework will facilitate not only enhanced sensitivity to discriminate between clinical conditions, but will also identify hypotheses about the likely mechanisms involved, which will be tested via causal manipulations using the same quantitative framework. Contribution to Overall Center Goals & Interactions with Other Center Components. Our modeling framework will be applied to data across all Projects, including measures of connectivity (P1), behavioral and neural activity (P2-5), clinical measures (P3- 5), and influences of neural (P2&5) and behavioral (P4) interventions. Cores B & C will help with localization and estimation of neural activity. We will benefit from interactions amongst experts with complementary expertise in systems and cognitive neuroscience, psychiatry, engineering, and computational modeling.