Functional Role of Corticogeniculate Feedback in Vision
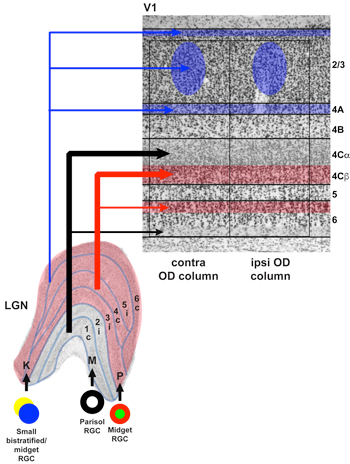 In mammals, all conscious visual information traverses a path from the retina through the visual thalamus (called the lateral geniculate nucleus or LGN) to the primary visual cortex (V1). Accordingly, in just a handful of synapses and in about 50 milliseconds, information about a visual stimulus in the world can reach the cortex. In highly visual mammals such as humans, visual information traversing this path is segregated into three parallel processing streams. By separating out visual information that is most relevant for processing motion, form/acuity, and color, signals can be relayed in parallel for increased processing speed. Even though parallel stream segregation is a hallmark of the early visual pathways in highly visual mammals, we are not aware of this separation of visual information because signals from the parallel streams are combined in the visual cortex. We tend to think about visual information encoding as a feedforward process in which information progresses from lower visual areas, such as the LGN and V1, to visual cortical areas higher up in the processing hierarchy. However, the circuits of the visual system include many feedback projections in addition to the more commonly studied feedforward projections.
In mammals, all conscious visual information traverses a path from the retina through the visual thalamus (called the lateral geniculate nucleus or LGN) to the primary visual cortex (V1). Accordingly, in just a handful of synapses and in about 50 milliseconds, information about a visual stimulus in the world can reach the cortex. In highly visual mammals such as humans, visual information traversing this path is segregated into three parallel processing streams. By separating out visual information that is most relevant for processing motion, form/acuity, and color, signals can be relayed in parallel for increased processing speed. Even though parallel stream segregation is a hallmark of the early visual pathways in highly visual mammals, we are not aware of this separation of visual information because signals from the parallel streams are combined in the visual cortex. We tend to think about visual information encoding as a feedforward process in which information progresses from lower visual areas, such as the LGN and V1, to visual cortical areas higher up in the processing hierarchy. However, the circuits of the visual system include many feedback projections in addition to the more commonly studied feedforward projections.
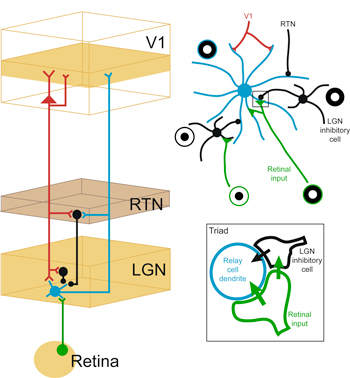 The first feedback circuit in the visual system is the corticogeniculate circuit connecting V1 to the LGN in the feedback direction. Corticogeniculate circuits are actually a subset of the larger family of corticothalamic circuits that link primary sensory cortex with the sensory thalamus in the feedback direction. As such, corticogeniculate circuits, and corticothalamic circuits in general, are thought to play an important role in regulating the feedforward flow of sensory information. In spite of many decades of study, the functional role of corticothalamic feedback in sensory perception has remained a fundamental mystery in systems neuroscience. Part of the difficulty in defining the functional role of corticothalamic feedback is due to the fact that these feedback circuits appear to merely modulate, rather than drive, changes in thalamic responses. In the visual system, corticogeniculate inputs onto LGN relay neurons far outnumber retinal inputs onto LGN relay neurons. However, LGN relay neurons have receptive field properties that mimic their retinal and not their cortical inputs. Are LGN relay neurons simply “relaying” (as their name would imply) retinal signals to the cortex, or is there some purpose for having a layover in the thalamus, midway between the retina and the cortex?
The first feedback circuit in the visual system is the corticogeniculate circuit connecting V1 to the LGN in the feedback direction. Corticogeniculate circuits are actually a subset of the larger family of corticothalamic circuits that link primary sensory cortex with the sensory thalamus in the feedback direction. As such, corticogeniculate circuits, and corticothalamic circuits in general, are thought to play an important role in regulating the feedforward flow of sensory information. In spite of many decades of study, the functional role of corticothalamic feedback in sensory perception has remained a fundamental mystery in systems neuroscience. Part of the difficulty in defining the functional role of corticothalamic feedback is due to the fact that these feedback circuits appear to merely modulate, rather than drive, changes in thalamic responses. In the visual system, corticogeniculate inputs onto LGN relay neurons far outnumber retinal inputs onto LGN relay neurons. However, LGN relay neurons have receptive field properties that mimic their retinal and not their cortical inputs. Are LGN relay neurons simply “relaying” (as their name would imply) retinal signals to the cortex, or is there some purpose for having a layover in the thalamus, midway between the retina and the cortex?
We have been dedicated to cracking the mystery of corticogeniculate feedback for many years. We previously discovered that corticogeniculate feedback is made up of multiple morphologically and physiologically distinct cell types (see Hasse et al., 2019; Briggs et al., 2016; Briggs & Usrey, 2005, 2007, 2009). Interestingly, these distinct cell types map precisely onto the distinct feedforward streams that are the hallmark of the early visual pathways. We hypothesize that corticogeniculate feedback is carrying stream-specific information from V1 back to the LGN. One of our current goals is to assess the functional connectivity between corticogeniculate and LGN neurons to see if functional connections support the stream-specific hypothesis for corticogeniculate feedback.
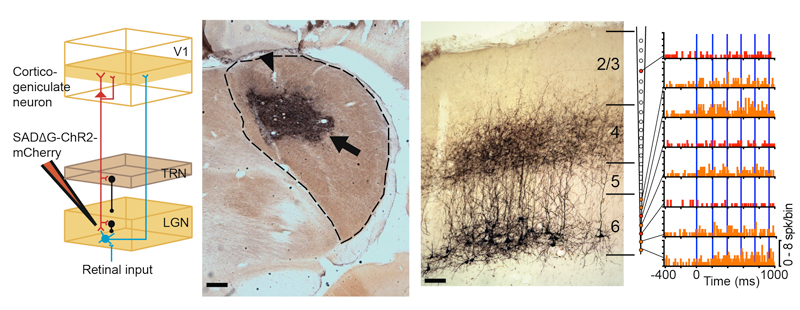
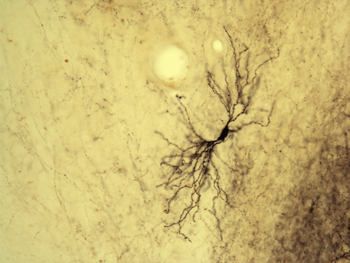 But what is the functional role of these feedback neurons? Recently, we utilized an innovative combination of virus-mediated gene delivery and optogenetics to selectively and reversibly control the activity of corticogeniculate neurons in vivo. We discovered that enhancing corticogeniculate feedback dramatically reduced the response latency of LGN relay neurons to incoming visual inputs (Hasse & Briggs, 2017). In addition to responding more quickly to visual inputs, LGN neurons also responded more precisely and their receptive fields narrowed. More recently, we discovered that optogenetic activation of corticogeniculate feedback reduces the response variability of LGN neurons and increases their capacity to convey information about visual stimuli. Interestingly, we did not observe any changes in the tuning properties of LGN neurons with enhanced corticogeniculate feedback. Our results therefore confirm that corticogeniculate feedback is not responsible for driving the response properties of LGN neurons. Instead, corticogeniculate feedback controls the timing and precision of LGN responses to incoming visual information. Future work will further explore whether any of these interesting effects on LGN response timing and variability are stream-specific.
But what is the functional role of these feedback neurons? Recently, we utilized an innovative combination of virus-mediated gene delivery and optogenetics to selectively and reversibly control the activity of corticogeniculate neurons in vivo. We discovered that enhancing corticogeniculate feedback dramatically reduced the response latency of LGN relay neurons to incoming visual inputs (Hasse & Briggs, 2017). In addition to responding more quickly to visual inputs, LGN neurons also responded more precisely and their receptive fields narrowed. More recently, we discovered that optogenetic activation of corticogeniculate feedback reduces the response variability of LGN neurons and increases their capacity to convey information about visual stimuli. Interestingly, we did not observe any changes in the tuning properties of LGN neurons with enhanced corticogeniculate feedback. Our results therefore confirm that corticogeniculate feedback is not responsible for driving the response properties of LGN neurons. Instead, corticogeniculate feedback controls the timing and precision of LGN responses to incoming visual information. Future work will further explore whether any of these interesting effects on LGN response timing and variability are stream-specific.
Most of our prior work has involved investigations of the role of corticogeniculate feedback at the level of individual neurons. We are also interested in exploring the effect of feedback on small populations of LGN neurons as population-level interactions are more likely to guide visual perception. For example, corticogeniculate feedback may play a role in synchronizing activity among small ensembles of LGN neurons. Patterns of synchronization are likely to depend on the stream-specific organization of corticogeniculate-LGN interactions, linking together a number of ongoing investigations in the lab.
An important extension of our current studies of corticogeniculate circuits is to move into the behavioral realm. We will perform selective corticogeniculate circuit manipulation using optogenetics or chemogenetics during visual behavior. In order to do that, ongoing work in the lab is exploring novel virus-mediated gene delivery methods that will support long-term behavioral studies. Based on our preliminary experiments, lentivirus pseudotyped with modified rabies glycoproteins can specifically infect corticogeniculate neurons retrogradely, and it also allows for long-term gene expression and survival of the infected cells.