Protective Adaptation of Articular Cartilage in Response to Mechanical Loads
Articular cartilage covers and protects the ends of long bones to enable smooth and pain-free joint motion. Within this important tissue, resident cells (chondrocytes) are responsible for maintaining the extracellular matrix (ECM). However, when cartilage is exposed to extreme mechanical forces, chondrocyte death can occur. For example, when the knee is destabilized by an injury to the anterior cruciate ligament (ACL), the resulting abnormal forces in the joint can initiate chondrocyte necrosis and trigger osteoarthritis (OA), a painful and complex joint disease characterized by progressive degeneration of cartilage and surrounding tissues. Similarly, cartilage viability is reduced by impact loads required to insert osteochondral (bone on cartilage) grafts during cartilage transplantation procedures to repair cartilage defects caused by injury or disease. Unfortunately, due to the low proliferative capacity of chondrocytes, widespread chondrocyte death has been implicated in the pathogenesis of osteoarthritis and in the failure of osteochondral transplants. Hence, a treatment that reduces the sensitivity of chondrocytes to mechanical forces has the potential to prevent the onset of OA following a joint-destabilizing injury or improve clinical outcomes for osteochondral grafting procedures
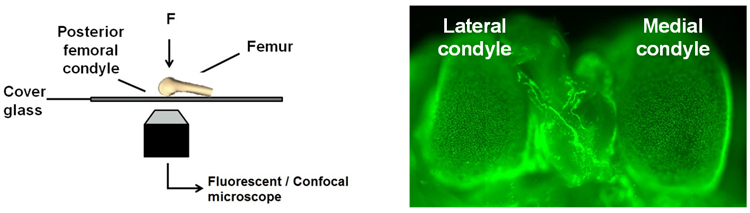
In our studies to date, we have employed a custom mechanical testing platform (Kotelsky et al., 2018) to demonstrate that the sensitivity of chondrocytes to mechanical forces can be controlled through their recent loading history, a phenomenon that we refer to herein as cytoprotective adaptation to mechanical stimuli (CAMS). That is, prior mechanical loading (mechanical preconditioning) of articular cartilage renders chondrocytes less susceptible to mechanically-induced cell death. By rigorously investigating the underlying mechanism of CAMS, we hope to identify new and promising targets of OA intervention. Furthermore, we propose to exploit CAMS by mechanically preconditioning osteochondral grafts to reduce risk of graft failure.