HBSR Policies and Procedures and Equipment
Human Biophysiology Shared Resource
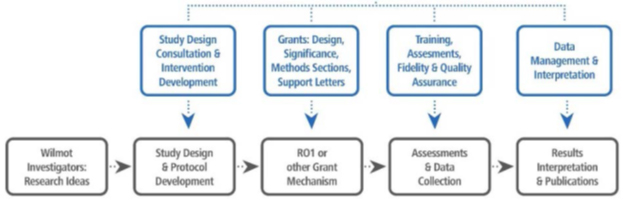
The Human Biophysiology Shared Resource utilizes expertise from the Cancer Control and Psychoneuroimmunology lab to support investigators at Wilmot Cancer Institute for biospecimen process, analyses of human biomarkers, human physiology assessment and integration of relevant cancer control clinical outcomes. We are a major shared resource for the Cancer Prevention and Control Program at Wilmot. All Principal Investigators, fellows and students who are interested in collaborating with the Cancer Control & Psychoneuroimmunology Laboratory are required to follow the guidelines of the University of Rochester’s Research Subject Review Board (RSRB).
If you are interested in the HSBR, please take the following steps:
- Schedule an appointment with Dr. Netherby-Winslow and appropriate CCPL laboratory staff to discuss projects and needs, and get study approval.
- Complete all CCPL laboratory based training before beginning any work within the laboratory can be done. (Any PI and any of the PI’s staff who will be working in the lab needs to complete the following as applicable to their research):
- Laboratory Safety Training by laboratory staff
- Certification for Greater Than Minimal Risk Biomedical Research
- EH&S Biological/Chemical/Animal Training
- Phlebotomists and Clinical Study Coordinators Training
- HIPAA Privacy and Security Training
- Hazard Communication and Global Harmonization Training
- If applicable, UCAR Training (consult UCAR office)
- Secure all University of Rochester approvals before beginning any work within the laboratory. Copies must be provided prior to initiating any work in the lab.
Guidance for Citation
Materials and Methods: All specifics of instrumentation and software used in analysis required for publication will be provided with data to users. Examples include:
- Example methods - All samples were analyzed on a Luminex Magpix (Luminex Corp., Austin, TX). The median of 50 beaded reactions per well was used to determine concentration per sample. Customized Milliplex xMAP human cytokine and cytokine receptor immunoassay kits (catalog numbers: XX); Luminex Corp.) were used for the study.
Acknowledgment: Sample processing, biomarker profiling, and consultation and on study design can be described as such:
- Example citation - We thank the Cancer Control Psychoneuroimmunology Lab (CCPL) within the Human Biophysiology Shared Resource (HBSR) at Wilmot Cancer institute for assistance with Luminex profiling and data interpretation and the for guidance in study design and protocol development.

Mark strawing clinical samples on the MAPI
Main Equipment and Services
The CCPL is a biosafety level 2 facility. We use multiple approaches to assessing biological outcomes (e.g. inflammation, adaptive immunity, angiogenesis, neurological function, metabolism, mitochondrial function) in various cancer populations and their caregivers. We routinely utilize ELISA, Luminex and other related assays based on standard operating procedures on clinical research samples (e.g. serum, plasma, DNA, RNA, saliva, tissue biopsies) in various cancer populations and their caregivers. The CCPL also assists investigators in the interpretation of assay results and provides assistance with interpreting biological outcomes in the context of psychological, clinical and biological outcomes, as well as moderation and mediation analyses, and correlative studies. Research supported by the lab include: mouse- to-human translation studies, pilot projects, Phase I-III randomized clinical trials, basic science modeling, and longitudinal epidemiological studies. The lab is located on the second floor at URMC within the Department of Surgery.
Available Equipment
- MAGPIX Luminex XMAP Technology
- Quanterix SR-X Biomarker Detection System
- Molecular Devices SpectraMax i3
- BrandTech Scientific, Inc. Liquid Handling Station
- QUIAGEN QIAcube
- The MAPI Machine and Domino printer to bank biological samples into micro-aliquot (100 μl, 300 μl, and 500 μl) from larger volumes of whole blood, plasma, or serum.


New Freezers
Biobanking in the CCPL
The Cancer Control and Psychoneuroimmunology Laboratory (CCPL) is the support laboratory for the URCC NCORP Research Base and houses all biobanking activities. The CCPL is a state-of-the-art clinical and translational research laboratory that investigates relationships between biological markers and effects of cancer, from cancer progression to side effect management from T1-T4 levels of research. The laboratory conducts biological sample collection, processing, analysis, and storage of multiple tissue types according to standard operating procedures.
Samples can be scanned via barcode scanners and entered in the Lab’s Freezerworks inventory management system. Freezerworks provides a centralized system that keeps track of every sample in one place, their precise location in freezers, when they were received, moved, used for testing. Lab managers can quickly view a map of all the freezers to evaluate available space, move sample in batches, run reports per study/participants/timepoints. Data is protected through individual user rights, the system keeps a comprehensive audit trail for all samples.
Blood Processing in CCPL
CCPL has developed SOPs for the collection and processing of liquid biospecimens, such as serum, plasma, whole blood (for DNA or RNA). Processing is available for microfuge tubes or straws for freezer or liquid nitrogen storage. Straws are processed in 2 mL, 1 mL, 0.5 mL, or 0.1 mL sizes; multiple aliquots from the same sample can be created to maximize analyses without freeze-thaws. Samples are linked to secure clinically annotated data.
When requesting scheduling blood processing in the CCPL, please follow the guidance outlined below.
Download:
Blood Processing Drop-off Form
Instructions
- Please create an Outlook Calendar Meeting for each Blood Processing Request
- Schedule a 60 Minute meeting starting at the estimated time blood will be dropped off at the CCPL Lab
- Invite Lab Staff to the Meeting:
- Add the Event to the CCPL Lab Calendar
- Send a follow up email notifying Lou Lotta that a calendar event had been created and an invitation has been sent
 Processing Information
Processing Information
- Include the following information in the body of the outlook invitation:
- Study Title:
- Study PI:
- Participant ID:
- Assessment #:
- Scheduled Date of blood collection:
- Scheduled Time of blood collection:
- Study Coordinator Name and Phone Number:
- Documents to Attach
- Study specific Blood Processing instructions
Any study specific blood processing guides should be delivered with each blood sample.