URMC / Labs / Dean Lab / Projects / Cytoplasmic Trafficking of DNA Effects of Strain on Gene Transfer
Cytoplasmic Trafficking of DNA Effects of Strain on Gene Transfer

Plasmids utilize the microtubule network for cytoplasmic movement during
transfection. When microtubules were disrupted by treatment with nocodazole,
cytoplasmically microinjected fluorescent plasmids (yellow) were unable
to move the nucleus.
While much of our focus over the years has been on how plasmids enter the nucleus, we recently turned our attention to the fact that there is a large distance between the plasma or endosomal membrane where DNA enters the cytoplasm and the nuclear envelope. Thus we asked the question of how plasmids move through the cytoplasm to even get to the nucleus. Using transfection, microinjection, and cell-free assays, we showed that plasmids move through the cytoplasm utilizing the microtubule network and its associated plus end-directed motor dynein. When microtubules are depolymerized or the activity of dynein is inhibited, gene transfer is also greatly inhibited. We have begun to identify the proteins involved and have found that the binding of certain transcription factors to the DNA can result in enhanced movement along microtubules. Further, plasmids that contain no binding sites for any transcription factor fail to traffic through the cytoplasm at all. We are currently beginning to identify other proteins involved in these trafficking complexes using a number of approaches including proteomics.

Barriers to gene transfer and processes affected by cyclic stretch.
Gene therapy is an important advancement with the potential to revolutionize clinical medicine. However, current technologies to deliver genes are not sufficient to make this a reality in patients under even optimum conditions, let alone under stresses associated with many disease states. Prolonged and even short-term exposure to mechanical ventilation can cause profound changes in the alveolar epithelium. We are trying to develop non-viral gene therapy approaches to treat the acutely injured lung. As such, we must understand the mechanisms of gene transfer in alveolar epithelial cells under conditions that mimic those found during lung injury and its management (i.e., ventilation). Mechanical stretch induces numerous biological responses in cells, including alterations in the cytoskeleton, activation of cell signaling pathways, and upregulation of transcription factors. Intriguingly, these responses are directly related to the process of gene delivery to cells and tissues.

Following transport of plamsids across the plasma membrane and translocation through the
cortical actin layer, plasmids associate with a number of DNA-binding proteins present in the
cytoplasm to form DNA-protein complexes.
Exogenous DNA, either viral or non-viral, must cross the plasma membrane into the cell, travel through the cytoplasm and the cytoskeletal networks, enter the nucleus, and be transcribed in order for gene therapy to be successful. Our lab has shown that plasmids utilize the microtubule network for movement through the cytoplasm and form complexes with transcription factors for their nuclear entry. Because mechanical stress alters both the cytoskeleton and the levels and subcellular localization of many transcription factors, it is likely that mechanical strain could play a large role in gene delivery. Indeed, we have observed that cyclic stretch greatly enhances gene delivery and expression in a wide variety of cell types, including those from the lung. We have found that this enhanced gene delivery is due to greater movement of the DNA through the cytoplasm, thereby allowing more DNA to reach the nucleus faster during a transfection.

Cyclic stretch increases gene transfer and
expression in lipoplex-transfected cells.
This occurs through the inhibition by stretch, of a cytoplasmic enzyme (HDAC6) that deacetylates tubulin, not histones. This inhibition results in microtubules that are more highly acetylated. These highly acetylated microtubules recruit more motor proteins and in turn bind more plasmid-protein complexes for movement through the cytoplasm to the nucleus. We are currently identifying the proteins that act as bridges between the plasmids and the microtubules and trying to understand how acetylation of the microtubules alters these complexes.
These experiments demonstrate that the cytoskeletal reorganization and transcription factor activation induced by mechanical stretch stimulate the ability of exogenous DNA, once inside the cell, to travel through the cytoplasm and into the nucleus for gene expression. Although not a physiologically “normal” process, the interactions of exogenous DNA with the host cell are vital to methods scientists use everyday and form the basis for the next paradigm of disease treatment: gene therapy. Because cyclic stretch of alveolar epithelial cells mimics the mechanical force induced by ventilators on the lung, we may ultimately extend our findings on stretch-induced increases in gene transfer to the lung in vivo to develop improved approaches for gene delivery. To this end, we have also shown in small animal models that by simply ventilating the lungs of animals immediately after electroporation-mediated gene delivery, or by inhibiting HDAC6 through small molecule inhibitors or by siRNA-mediated silencing, we have greatly increase gene delivery and expression to the lung. This is yet another example of increasing gene delivery in animals by studying very simple and basic mechanisms of the cell biology of the gene transfer process.
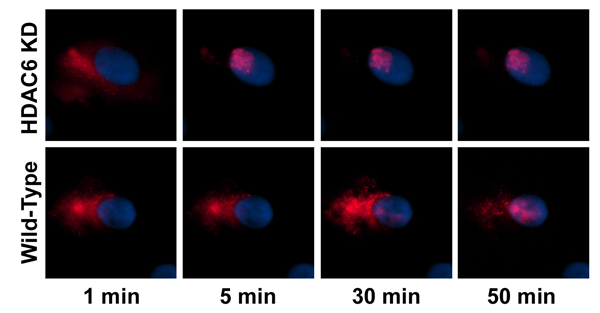
Inhibition of HDAC6 increases cytoplasmic trafficking of plasmids. Wild type or HDAC6-knock down cells were
cytoplasmically microinjected with fluorescently labeled plasmids and their subcellular localization was followed
over time. Cells with reduced HDAC6 activity (and increased levels of acetylated microtubules) showed much
faster nuclear localization of the injected DNA.
« back to all projects