Research Projects
Prostate Cancer
Prostate cancer is the most common cancer in men, with approximately 230,000 new cases diagnosed in the United States every year. In fact, it is estimated that 1/7 men will be diagnosed with prostate cancer. Most patients with prostate cancer have localized disease that does very well. Simple surgery of external beam radiation can cure the vast majority of these localized cancers. However, 5-10% of patients with prostate cancer have advanced disease which carries a dismal 30% five-year survival rate. While there are several therapies that are currently being used to treat patients who have advanced disease, including androgen deprivation and chemotherapy, these approaches ultimately fail. Tumors become “castration resistant,” at which point their growth is nearly impossible to stop. Thus, finding new targeted therapies that will treat both androgen-dependent and castration-resistant prostate cancer is extremely important. Our laboratory has taken two approaches toward this problem, as described in the two projects below.
The Role of Paxillin in Prostate Cancer Progression
In approximately 2005, our laboratory made an interesting discovery regarding the scaffold molecule called paxillin. At that time, paxillin had been traditionally thought of as a cytoplasmic regulator of cell-cell adhesion and cytoskeletal rearrangements. However, using a model system of androgen-induced meiosis in Xenopus frog oocytes, we demonstrated that in fact paxillin also acts as an important regulator of extranuclear, or nongenomic, androgen receptor signaling. In subsequent years, we showed that paxillin similarly regulates extranuclear androgen signaling in prostate cancer. In fact, we found that paxillin is a critical liaison between extranuclear and intranuclear androgen receptor signaling in prostate cancer cells, serving both as a regulator of androgen-triggered kinase signaling as well as a modulator of transcription. Further discoveries broadened the role of paxillin in prostate cancer progression. We now have shown that paxillin does not just regulate androgen receptor signaling in the nucleus – it also mediates MAPK signaling both outside and inside the nucleus, regardless of the stimulus. Paxillin is upregulated in prostate cancer, and we find that paxillin favors proliferation, migration, and invasion of prostate cancer cells in response to a number of signals, including androgens and growth factors. Therefore, the paxillin pathway may serve as an important target for the treatment of both hormone-sensitive and castration-resistant prostate cancer. With this in mind, our laboratory continues to use genomics, in-vitro models, mouse xenograft models, and human prostate cancer samples to further our understanding of paxillin’s role in prostate cancer.
Inflammation and Prostate Cancer
As our laboratory began to focus on prostate cancer biology, our attention turned to “other” types of cells that are associated with the epithelial prostate cancer cells. In nearly all cancers, the tumor cells are intermixed and/or surrounded by “stromal” cells, which consist of fibroblasts, endothelial cells, immune cells, and many other cell types. It is very clear now that all of these stromal cells play a critical role in the growth and progression of cancer. Our laboratory has chosen to focus on the immune cells. We normally think of the immune system as “protector” for our bodies; its job is to eliminate foreign or bad objects from our bodies. However, cancer cells have learned how to use our immune system against us, and inflammation has now been linked to the progression of many different cancers. We are interested in how a particular class of immune cells, called neutrophils, and their related myeloid-derived suppressor cells (MDSCs), promote prostate cancer growth and metastasis. We have taken a particular interest in an enzyme that is secreted from neutrophils and some MDSCs called neutrophil elastase, as we have evidence to suggest that this enzyme may be a critical promoter of prostate cancer cell proliferation, migration, invasion, and metastasis. We use mouse knockout models, human xenograft models, in-vivo imaging strategies, and in-vitro systems to study how these granulocytic cells promote prostate cancer growth. We are specifically interested in utilizing inhibitors of neutrophil elastase in our studies, as these drugs, which are already being tested in humans for other inflammatory disease, may prove useful in cancer treatment as well. Our ultimate goal is to discover novel targets that we can manipulate in patients to mitigate the negative actions of inflammation on prostate cancer progression.
Estrogen, Inflammation, and Lymphangioleiomyomatosis (LAM)
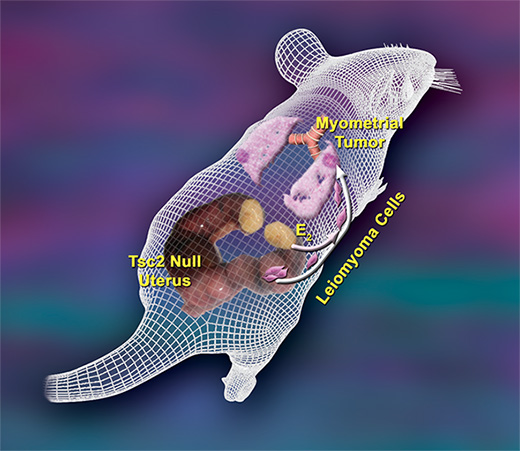 Since approximately 2010, our laboratory has been interested in the rare disease called lymphangioleiomyomatosis, or LAM. LAM is a progressive cystic lung disease that often leads to lung failure, sometimes requiring lung transplantation. Interestingly, the cystic lung disease is caused by a myriad of small smooth muscle cell tumors that invade the lung and destroy normal lung tissue. Therefore, while LAM is taken care of primarily by pulmonologists, it is in fact a cancer. LAM has several interesting features that include the following:
Since approximately 2010, our laboratory has been interested in the rare disease called lymphangioleiomyomatosis, or LAM. LAM is a progressive cystic lung disease that often leads to lung failure, sometimes requiring lung transplantation. Interestingly, the cystic lung disease is caused by a myriad of small smooth muscle cell tumors that invade the lung and destroy normal lung tissue. Therefore, while LAM is taken care of primarily by pulmonologists, it is in fact a cancer. LAM has several interesting features that include the following:
- LAM is found almost exclusively in women;
- LAM cells contain mutations in one of the two tuberous sclerosis (TSC) tumor suppressor genes, which results in activation of the mTORC1 proliferative pathway (hence mTOR inhibitors can be used to treat LAM in patients);
- LAM cells appear to be very sensitive to estrogen in most in-vitro and mouse models, as well as in patients; and
- LAM cells express markers normally found in melanocytes, suggesting some kind of similar origin.
- LAM lung tumors appear to be metastatic, as patients with advanced disease who receive lung transplantations often have recurrence of the disease in the new lungs.
Our laboratory has created a knockout mouse model for LAM which we are using to uncover novel mechanisms that regulate LAM tumor cell growth. In this model, we knocked out Tsc2 in the mouse uterus, which leads to the formation of smooth muscle cell tumors in the uterine myometrium that share most characteristics of human LAM. In addition, we see metastasis of uterine smooth muscle cell tumors to lungs, making our mouse the first metastatic model for LAM. Together, our model of metastasis from the uterus offers a possible explanation for both the origin of the LAM cell as well as the incredible female sexual dimorphism seen in the disease. With our background in steroid hormones, we are particularly interested in studying the role of estrogen in mediating LAM cell growth; we believe that estrogen may be a critical regulator of LAM progression and may serve as an important target in the treatment of LAM. In addition, we are studying the melanocyte markers and how they might contribute to LAM progression. Using our mouse models, we have discovered that the melanocytic protein GPNMB is a new biomarker for LAM, as well as a potential drug target. Finally, our laboratory is interested in studying the role of inflammation in LAM progression. Our goal is to uncover novel approaches that we can use to treat this rare but devastating disease.
Androgen-Mediated Regulation of Ovarian Function and Female Fertility
While estrogen and progesterone are traditionally thought to be the “female” sex steroids, recent evidence indicates that androgens such as testosterone are also very important in female physiology. Specifically, our laboratory focuses on androgen actions in the ovary. We find that androgens are critical regulators of follicle growth and development, functioning primarily, but not necessarily exclusively, via androgen receptors in granulosa cells. We find that loss of androgen signaling in granulosa cells leads to reduced follicle development, decreased ovulation, and eventually Diminished Ovarian Reserve (DOS). In contrast, androgen excess, as seen in the common disorder Polycystic Ovary Syndrome (PCOS), leads to excessive and unregulated follicle growth, which ultimately results in decreased ovulation and reduced fertility. We find that androgens signal through the androgen receptor in both extranuclear (nongenomic) and intranuclear (genomic or transcriptional) fashions to modulate ovarian follicle development and eventual ovulation. Our laboratory is focused on continuing to investigate how and where androgens signal in the ovary to modulate follicle health, with the long-term goal being to design potential interventions that will modulate androgen signaling and perhaps improve fertility in women with Diminished Ovarian Research or Polycystic Ovary Syndrome.