Cellular Mechanisms of Metabotropic Glutamate Receptor Dimer Formation
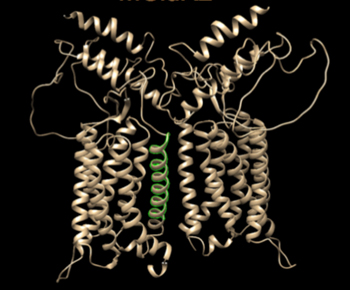
mGluRs function as stable, covalently linked dimers. This means that a functional receptor consists of two mGluR proteins. The proteins can either be identical, called a homodimer, or different, called a heterodimer. While all mGluRs can form homodimers, several can heterodimerize with some other mGluR proteins, but this process is not promiscuous. Only certain combinations of mGluRs can heterodimerize. Moreover, the organization of mGluRs into heterdimers can profoundly alter how the functioning receptors respond to pharmacological ligands. But although it is now clear that the dimeric state is extremely important to know if we are to understand how modulatory compounds will affect neural function in areas where the targeted receptors are expressed, very little is know about how dimer selectivity arises in cells. We are interested in the cell biological processes that lead to mGluR dimer formation and whether each receptor forms predominantly homo-or heterodimers in a particular cell.
Important questions:
- Is there selectivity in binding between mGluR N-termini, the extracellular portion of the proteins that get linked by a disulfide bridge during protein synthesis in the endoplasmic reticulum?
- Do the intracellular C-termini of mGluRs possess verifiable ER retention sequences that prevent trafficking to the plasma membrane unless masked by binding to the C-tail of another receptor?
- Do the transmembrane spanning regions of mGluRs play a role in selective association of mGluRs during protein synthesis and thus contribute to heterodimer selection?