Sumoylation and Aging
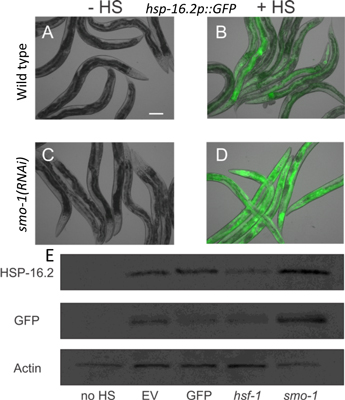
Figure 1. (A-D) Hyper-induction of Phsp-16.2::GFP in response to heat shock in sumoylation-defective animals. See Das et al. (2017) PLoS Genetics for additional supporting experiments.
The mechanisms that maintain proper function and folding of the proteome (proteostasis) decline during normal aging, which facilitates the onset and progression of neurodegenerative protein misfolding diseases, including Alzheimer’s Disease. The functional integrity of the proteome is safeguarded from stress through the combined action of a cohort of transcription factors, each primed to respond to specific forms of proteotoxic stress. During aging, these responses decline and ultimately precipitate a collapse of proteostasis. C. elegans is an excellent model to study the molecular mechanisms involved in this complex process: in particular, the inducibility of the heat shock response, mitochondrial unfolded protein response, ER unfolded protein response, and the oxidative stress response all rapidly decline concurrent with early signs of declining proteostasis. Why the inducibility in response to diverse forms of proteotoxic stress declines, however, is poorly understood, but coincides with the formation of repressive chromatin marks at stress loci.
We have identified inappropriate sumoylation during aging as a potential mechanism to explain loss of stress response inducibility. For instance, we have previously shown that knockdown of the sole C. elegans gene encoding the SUMO moiety enhances the heat shock response (Figure. 1 and Das et al. 2017 PLOS Genetics). Conversely, we previously discovered that preventing deSUMOylation, by knockdown of the SUMO isopeptidase ulp-1, shortens lifespan (Samuelson et al. 2007 Genes and Development).
This project will explore how changes in sumoylation during aging alter the epigenome, inducibility of stress responses, and maintenance of proteostasis. The objectives of this project are to identify nuclear changes in sumoylation during aging and gain mechanistic insight into how altered sumoylation intersects with changes in chromatin, inducibility of stress response, and the consequence on proteostasis and longevity. Many of the causative factors of neurodegenerative diseases are sumoylated and mutations in core components of the sumoylation machinery are found in AD patients. Thus, elucidating the intersection between sumoylation, chromatin, and the proteostatic network may have implications for the treatment of neurodegenerative disease and efforts to improve healthy aging.