Skin Graft Rejection & Whole-mount Histology
Skin graft rejection is a well-established technique in X. laevis to determine the segregation of both major and minor H-antigen (Ag) differences (Du Pasquier & Bernard, 1980; Ramanayake et al., 2007). Briefly, a piece of ventral skin (5x5mm) from a donor is inserted under the dorsal skin of a recipient and 24 hrs later the overlying host skin is removed. The time for complete skin rejection is determined by the time required for the destruction of all silvery iridophore pigment cells in the grafted skin (Fig. 8). At 21-22ºC, a recipient rejects donor skin displaying a 1 or 2 MHC haplotype mismatch subacutely within 18-22 days in X. laevis. In contrast, skin grafts from MHC identical donors that differ only by minor H-Ags are rejected more slowly (from 30 to 200 or more days). No rejection at all occurs when donor and recipient are genetically identical (e.g., fully inbred). This technique, therefore, is very powerful for both characterizing MHC combination and the degree of inbreeding, with the advantage of keeping both the donor and the recipient alive. It is also a very convenient method to assess T cell responses. For example, priming LG-6 with the heat-shock protein gp96 derived from minor H-Ag-disparate LG-15 tissue induces an accelerated rejection of LG-15 skin graft as compare to control recipient injected with cognate LG-15-derived gp96 or unprimed control (Robert et al., 2004). Whereas adult MHC-disparate skin grafts are rejected by larvae, minor H-Ag-disparate skin grafts are fully accepted (Fig. 6D), and remain permanently in the adult recipient after metamorphosis. This allotolerance to adult skin graft from minor H-Ags mismatched donor is Ag-specific since it induces permanent survival of a second-set skin graft from the same H-Ag mismatched donor that is grafted after metamorphosis but not a third-set skin graft from a different minor H-Ag-disparate donor. (reviewed in Robert and Ohta, 2009)
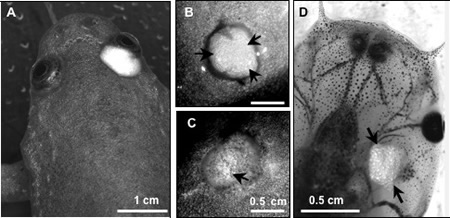
X. laevis adult recipient MHC-homozygous inbred of the J strain received a skin graft from either a MHC-identical (A, B) or a MHC-disparate outbred donor (C). Note the disappearance of the silvery pigments in the MHC-disparate rejected, but not MHC identical skin graft (Arrow). (D) Absence of rejection of adult LG-6 skin graft (arrows) by a pre-metamorphic LG-15 larva that differs at multiple minor histocompatibility loci. X. laevis adult recipient MHC-homozygous inbred of the J strain received a skin graft from either a MHC-identical (A, B) or a MHC-disparate outbred donor (C). Note the disappearance of the silvery pigments in the MHC-disparate rejected, but not MHC identical skin graft (Arrow). (D) Absence of rejection of adult LG-6 skin graft (arrows) by a pre-metamorphic LG-15 that differs at multiple minor histocompatibility loci.
To better define the effector cells involved in the immune response to skin allo-antigens of X. laevis, we have adapted a whole-mount immunohistology procedure used in mice that enables us to visualize leukocyte infiltration into unfixed transplanted skin tissues using fluorescent antibodies (Ramanayake et al., 2007). This is a very convenient, powerful and simple technique. It does not require fixation, clearing or embedding, and, therefore, it preserves inherent tissue architecture and protein conformation, including antigenic determinants. The skin graft surgically removed at different time following grafting, allows one to monitor in detail different leukocyte populations by direct incubation with X. laevis-specific mAbs, such as anti-CD8 mAb (Fig. 9).
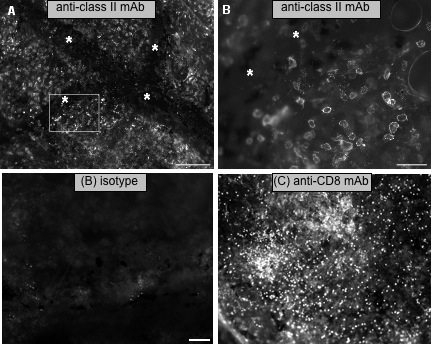
(A) Leukocyte infiltration in the vicinity of blood vessels (*) of MHC-disparate X. laevis skin undergoing rejection, 7 days post-transplantation stained with anti-class II mAb.Scale bar = 200 microns. (B) Magnification of the boxed area. Scale bar = 50 microns (adapted from Robert et al. 2008)
(B, C) CD8 T cell infiltration in minor H-Ag-disparate skin undergoing rejection. Whole-mount immunohistology of LG-6 skin tissues 12 days posttransplantation stained for mouse IgM isotype control (B) or X. laevis-specific anti-CD8 mAb AM22 followed by a PE-conjugated mouse mu-specific Ab (C). LG-6 skins were grafted onto LG-15 recipients 1 wk after adoptive transfer of LG-15 PLs (500,000) that were pulsed 1 h with 800 ng of LG-6-derived gp96. Whole-mount immunohistology of each transplant was performed 12 days postgrafting (30-35% rejection). Scale bar, 50 microns (adapted from Robert et al. 2008)