Clinical History
A 69-day-old male twin infant (corrected gestational age: 38 2/7 weeks) was delivered at 28 4/7 weeks gestation via cesarean section for advanced preterm labor and breech-breech position. He was the second twin of a monochorionic/diamniotic twin gestation. There was no maternal history of tobacco, alcohol, or illicit drug use. There were no definitive signs and symptoms of chorioamnionitis. The mother is a 27-year-old G2P0010.
At birth, the infant weighed 1170 grams with a crown-heel length of 38.5 cm and a head circumference of 26.5 cm. Each of these values was appropriate for gestational age. APGAR scores were as follows: 4 (1 minute) and 6 (5 minutes). He was cyanotic and depressed with a weak cry at delivery and was treated with intubation and surfactant administration following a trial of blow-by oxygen.
The child’s Neonatal Intensive Care Unit (NICU) stay was complicated by many medical conditions associated with prematurity, including: grade 3 intraventricular hemorrhage (IVH) with anemia and posthemorrhagic hydrocephalus, bronchopulmonary dysplasia, and feeding intolerance due to a presumed septic ileus.
Six weeks into his course this infant became pallid and febrile, and blood cultures revealed a Staphylococcus aureus sepsis that was treated with nafcillin and gentamicin. A heart murmur heard on physical examination led to the performance of an echocardiogram, which showed mitral regurgitation. A subsequent echocardiogram disclosed an endocarditis with vegetations affecting the mitral valve and left atrial septum.
Due to worsening mitral regurgitation and declining cardiac function, a mitral valve replacement was performed. The child&rsqup;s condition continued to decline post-operatively—he was diagnosed with renal failure secondary to congestive heart failure. Five days after his surgery, ST elevation was noted on electrocardiogram (ECG). The infant’s parents later requested that life support be withdrawn.
Gross Examination
Gross examination of the infant’s heart showed localized exudate on the heart surface over the distal left anterior descending coronary artery. This pericarditis was consistent with the child’s median sternotomy and mitral valve replacement procedure. The sternum was left open post-operatively and yellow exudates noted overlying the surgical site, which grew 2+ Pseudomonas aeruginosa upon culturing.
A 12 mm Carpentier-Edwards porcine bioprosthesis was identified at the left atrio-ventricular junction (Figure 1). A right atriotomy sutured incision was also noted, with adjacent subendocardial hemorrhage in the regions of the atrioventricular (AV) node and membranous septum. The infant’s left atrial appendage was dilated, thinned and darkened and contained an antemortem thrombus.
Additionally, there was epicardial mottling of the interventricular septum and the anterior wall. A left ventricular cleft was evident grossly (arrow, Figure 2), extending from the endocardium through the myocardium and ending approximately 2 mm from the epicardial surface.
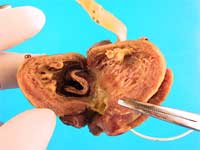
Figure 1
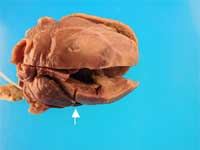
Figure 2
Microscopic Examination
The placental histology of the dividing membrane confirmed a monochorionic diamniotic twin gestation (Figure 3 shows dividing membrane roll with back-to-back amnions and no chorion). There was also a possible acute/recent twin-twin transfusion from Baby “A” to Baby “B” (our patient). The villi from disc “A” were edematous (Figure 4), and those from disc “B” were congested (Figure 5).
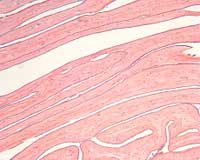
Figure 3
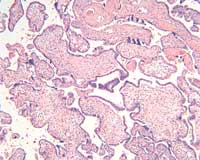
Figure 4
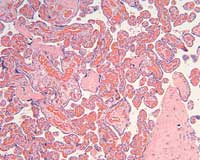
Figure 5
There was subendocardial and myocardial hemorrhage throughout the right atrial wall and proximal to the left main bundle branch of the conduction system. There were also foci of anucleic myocardium, necrosis, hemorrhage, and calcification (Figures 6-7).

Figure 6
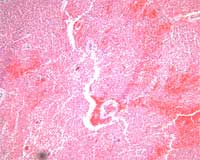
Figure 7
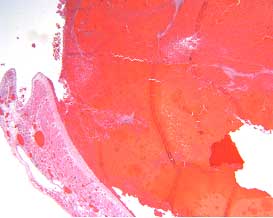
Figure 8
Histology of the left atrial appendage showed organized thrombus (Figure 8).
Microscopic evaluation of the interventricular septum and left ventricular anterior wall revealed subepicardial hemorrhage adjacent to regions of anemic infarction and patchy myocardial necrosis. The left ventricular cleft was surrounded by infarcted and necrotic myocardium with development of granulation tissue (Figures 9-10).
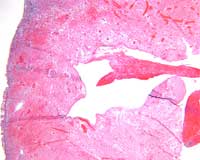
Figure 9
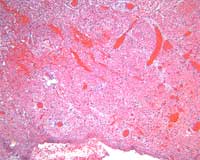
Figure 10
Discussion
Left ventricular free wall rupture is a relatively common finding in patients who die with an acute myocardial infarction (14 to 26 percent of these individuals have notable cardiac rupture), though the incidence of rupture is less that 1 percent when all patients who have had an acute myocardial infarction are considered [1-3]. The anterior and lateral walls of the left ventricle—near the junction of the infarcted and normal myocardium—are most commonly affected. In addition, a number of pathologic patterns are observed depending, in part, on the time that rupture occurs [4].
Myocardial rupture typically occurs near the boundary of infarcted and normal myocardium. It can be seen within the first five days after infarction in approximately 50 percent of cases and within two weeks in over 90 percent of cases [5]. An abrupt slit-like tear that perforates a necrotic area of myocardium characterizes early rupture, defined as less than 72 hours from acute infarction; anterior sites are preferentially affected. Late ventricular rupture—greater than four days from the acute event—can be characterized by infarct expansion—thinning and a disproportionate dilatation within the softened necrotic zone—without a preferred location.
The Multicenter Investigation of Limitation of Infarct Size (MILIS) study has identified certain risk factors for myocardial rupture. In addition to thrombolytic therapy, such factors include: no history of previous angina or myocardial infarction, ST-segment elevation or Q wave development on the initial ECG, peak MB-creatine kinase > 150 IU/L, an anterior location of the infarction, age > 70 years, and female sex [2].
The relevance of this epidemiologic data to this infant is limited, as the literature on pediatric myocardial infarction and subsequent myocardial rupture is sparse. The pathologic findings in this child, however, are consistent with an imminent late ventricular rupture at the boundary between healthy and necrotic myocardium.
The most common cause for bacterial endocarditis in children with normal hearts is Staphylococcus aureus [6, 7]. Congenital heart disease, especially tetralogy of Fallot, is a major risk factor for endocarditis [6]. The complications of endocarditis in children without congenital heart disease have been reported as congestive heart failure, central nervous system damage, and aortic regurgitation, but not myocardial rupture or infarction [6]. Myocardial rupture due to Staphylococcus aureus septicemia and endocarditis has been reported in adults but is rare [8]. Left ventricular rupture after mitral valve replacement has been described in an adult [9] but no references to myocardial rupture after valve replacement in children was found in the literature.
Abigail L. H. Kroening, Pathology Student Fellow, 2005-2005
References
- Stevenson WG, et al. Am Heart J 1989; 118:1182.
- Pohjola-Sintonen S, et al. Am Heart J 1989; 117: 809.
- Becker RC, et al. J Am Coll Cardiol 1999; 33: 479.
- Purcaro A, et al. Am J Cardiol 1997; 80: 39.
- Batts KP, et al. Hum Pathol 1990; 21: 530.
- Johnson DH, et al. Circulation 1975; 51: 581.
- Tolan RW Jr, et al. Clin Infect Dis 1992; 14: 852.
- Osula S, et al. Heart 2001; 85: e4.
- Bauerschmitt R, et al. Ann Thorac Surg 1998; 65: 359