Current Research Projects
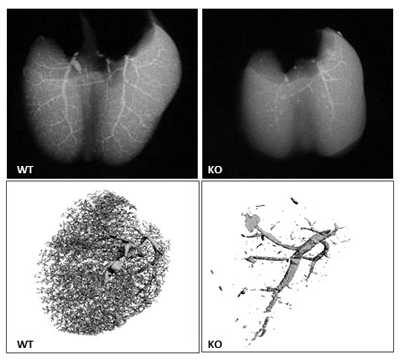
Imaging shows reduced vasculature in lungs of
GIT1 KO mice.
Angiogenesis, the formation of new blood vessels from existing ones, is a critical event for tissue development and repair, as well as being associated with many diseases (e.g. bronchopulmonary dysplasia(BPD), pulmonary artery hypertension (PAH), ischemic cardiomyopathy, retinopathy and tumor growth). Newly formed and pre-existing vessels are continuously under dynamic remodeling (including vessel regression, neointima formation and artery-vein specification) to accustom to changes of mechanic force, oxygen level, metabolites and inflammatory factors et al. The long-term goal of Dr. Pang’s lab is to illustrate the molecular mechanisms of angiogenesis and vascular remodeling under physiological and pathological conditions.
1.The molecular mechanism of PAH progression.
PAH is a progressive disease, characterized by vasoconstriction and cell proliferation, leading to elevated pulmonary arterial pressure and often causing right heart failure and death. Elevation of plasma cytokines in PAH patients is a hallmark of inflammation. As the major effector, monocytes release cytokines and infiltrate in perivascular regions of the lung. Depletion of monocytes attenuates vascular remodeling and hemodynamic changes in PAH animal models. This evidence implies communication between monocytes and endothelial cells (ECs). We are studying several signaling pathways that are important for the communication. In addition, we closely work with clinicians to identify human genetic mutations that cause PAH and study the related mechanisms using animal models.
2. The role of lung endothelial progenitor cells (LEPCs) in BPD.
BPD is a common disease afflicting premature newborns who receive mechanical ventilation and oxygen therapy. The pathology of BPD is consistent with an “arrest” of lung development, characterized by impaired angiogenesis, alveolarization and increased fibrosis. Endothelial progenitor cells (EPCs) have the capacity to differentiate to mature ECs to promote neovascularization or repair injured vessels. Clinical studies demonstrated decreased circulating EPCs are associated with BPD. Recent studies found that residential LEPCs are much higher than circulating EPCs, implying an important role of LEPCs in lung vascular function and disease. We identified two genes by combined screening of DNA methylation and transcriptome analysis, which may play important roles in LEPCs differentiation and propagation. We are generating transgenic mice to study the molecular mechanisms of LEPC function during development of BPD, and we also collaborate with LungMAP groups in our university to confirm our findings in BPD human samples.
3. Generating vascular imaging analysis system using artificial intelligence (AI) technology.
Mouse retinal vasculature is a well-recognized angiogenesis model in which the dynamic vascular plexus formation can be visualized by whole mount staining of endothelial markers. Pang’s lab uses this model to study the molecular mechanisms in vivo. Images of mouse retina after staining contain amazingly rich information and demonstrate dynamic changes of vasculature during development. We are collaborating with colleagues from Department of Statistics to generate a software using deep learning methods. Novel geometric and topological features of vessels will be extracted for evaluating vasculature development and disease progression using this powerful and precise tool.