For immediate cell sorting assistance the staff office can be reached at x6-5330.
There are three cell sorters available for investigators to choose from. The two FACSAria II sorters have near identical configurations (4 lasers each, Waldorf has one more violet detector than Statler) capable of managing panels approaching 18 colors.
Our Aurora CS is a full spectrum sorter with 5 lasers, capable of managing panels approaching 40 colors.
Be sure to review each sorter’s configuration page (linked above) before choosing an instrument. If you have any questions or concerns about choosing a sorter that’s right for you or wish to discuss anything in more detail, please contact a staff member so we can address your needs.
Sort Speed, Nozzle Size, and Cell Size
The default configuration of the FACSAria II uses an 85 micron nozzle. We have found that this nozzle configuration works very well for most of the cell types we sort – from lymphocytes to a multitude of cell lines. The Aurora CS default configuration uses a 100 micron nozzle.
If you have a larger cell type or need a gentler sort we can switch to a 100 micron nozzle (the 130 micron nozzle is rarely used and only necessary for very large cells or very low pressure sorts. Only the Arias offer the option of the 130 micron nozzle.) Switching to the 130 micron nozzle can take up to 30 minutes in order for the fluid stream to stabilize. In light of this, if you are requesting a 130 micron nozzle sort, please remember to build an extra 30 minutes into your reservation to allow for this during nozzle changeover.
Smaller cell types or bacteria can be sorted using the 70 micron nozzle.
The largest determining factor in selecting a nozzle size is the size of your cells. As a general rule, your cells should be no larger than one quarter the diameter of the nozzle with which you are going to sort. The fragility of the cells to be sorted is another significant factor. Fragile cells should be sorted at lower pressures.
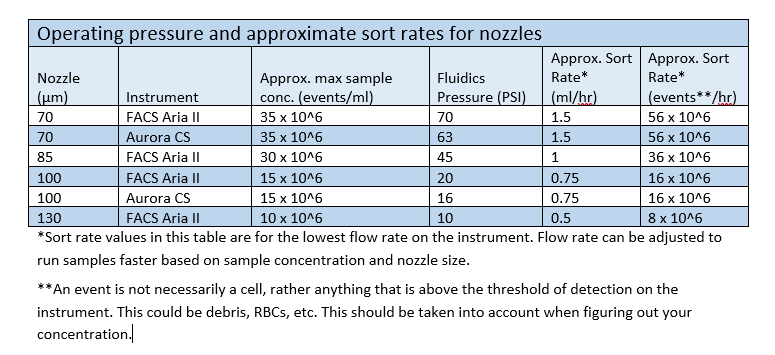
We encourage investigators unsure of which nozzle size to select to provide a small sample of their cells a week before the proposed sort to allow us to determine which nozzle to use.
Sort Precision Modes/Sort Masks
Our sorters have various Precision Modes to choose from, which will affect the way the machine sorts cells.

Proper Controls and Supplies
Adequate controls and supplies help ensure a quality sort.
What to bring with you for your sorting experiment:
- Unstained Cells/Negative control – so we can establish the size and fluorescent background of your cells.
- Single Stained Controls – For each fluorochrome you are using. These can be either beads (“Simply Cellular Compensation Standard” beads from Bangs Lab, “BD CompBeads” from BD Biosciences, etc.) or cells. It is important that you have a positive and negative in your control samples. Likewise, if you use beads for compensation, please make sure to provide a negative (unstained) bead as well. **If you have questions about compensation, compensation controls, or experimental controls, please contact the Flow Core staff for assistance!**
- A known positive control can be useful for reference if possible.
- Your sample at an appropriate concentration for the nozzle being used
- Sufficient collection tubes for the cells you wish to sort
- Extra media/buffer with which to dilute your sample or make up new collection tubes.
- Please use a container with a locking (or zipping) lid to transport samples to and from the Flow Core in keeping with Biosafety regulations!
Collection Vessels
The Arias can collect up to four sorted populations in 1.5mL and 5mL tubes and up to two sorted populations in 15mL tubes. The Aurora CS can collect up to six sorted populations in 1.5mL or 5mL tubes and up to two sorted populations in 15mL tubes.
The Arias can also use various sized plates for collection. Those plates must be (or have the same form factor as) BD Falcon Polystyrene plates with 6, 24, 48, 96, or 384 wells. The Aurora CS can sort into 96 or 384 well plates.
Sorting onto glass slides— frosted or unfrosted— is also possible with the Arias.
Sorting bulk amounts or single cells can be accomplished for plates and slides.
Collection Vessel Capacity
Media in collection tubes should be chosen based on the needs of the sorted cells. Sorting for continuing culture will be very different than sorting for downstream genomics work. There are no specific requirements from the FCR for collections media. The suggested minimum appropriate volume of media for a collection tube is 10% of the volume of the tube (i.e. 500uL in a 5mL tube), however that can be adjusted if necessary.
It is important to pre-coat collection tubes with media containing serum to increase post-sort viability and recovery. This involves coating the entire inner surface of the collection tube. Sorted cells that hit bare plastic have the potential to stick and dry out or burst.
To aid in the recovery of a rare sort population of low percentage in the sample, a high volume of media in a conically shaped collection tube is recommended (750uL in a 1.5mL eppendorf).
Collection Tubes can hold (approximately) the following number of cells based on the nozzle used. These numbers are based on collection tubes that have 10% of the volume as collection media.
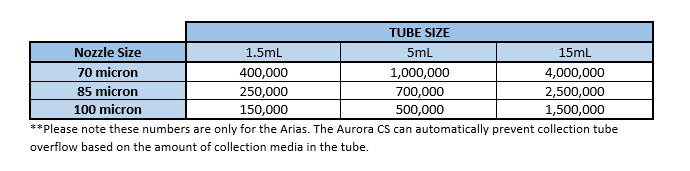
The figures in the above table can be influenced by the sort precision mode that is chosen as well as the frequency and amplitude with which droplets are being generated (on the Arias only, which changes droplet volume.) If an Investigator thinks modifying the droplet size will be beneficial to their sort or simply wishes to know more about the method they should inquire with the Flow Core staff. The approximate droplet volume for the standard 85um nozzle configuration is 2.5nL.
A Note on Adherent Cells
Adherent cells pose a special problem for a sorter. In general these cells have been treated with trypsin to remove them from the culture plate. Standard practice is to then add serum to neutralize the trypsin, however, this causes problems as trypsin adds back diavalent cations necessary for cells to adhere to each other. **It is better to use a trypsin inhibitor to inactivate your trypsin rather than serum**. Adherent cells, activated cells and cells with extensive structures outside the cell membrane all benefit from a slower sort (lower sample concentration, lower flow rate.)
DNAse in Sorting
As cells die they can release their DNA into the sample buffer. This released DNA readily binds to other cells causing clumping and reducing the effectiveness and quality of the sort. The addition of 10 Units of DNAse per mL of sample will help prevent this effect. This is especially important with adherent cells.
Fee Structure
Sort rates include a setup fee and an hourly charge with a 1 hour minimum; time is then billed to the minute. Time billed will be time reserved unless the reservation runs long, then the extra time will be added to the bill. Bills are sent out at the end of the month.
Please direct all billing questions to Matt Cochran.
Investigators that cancel less than 48 hours before the start of the scheduled sort time will be charged for the time unless the reservation is after 5pm, in which case a charge will be applied.