Cell Deformability & Cell Adhesion
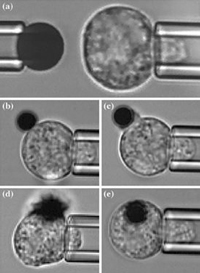
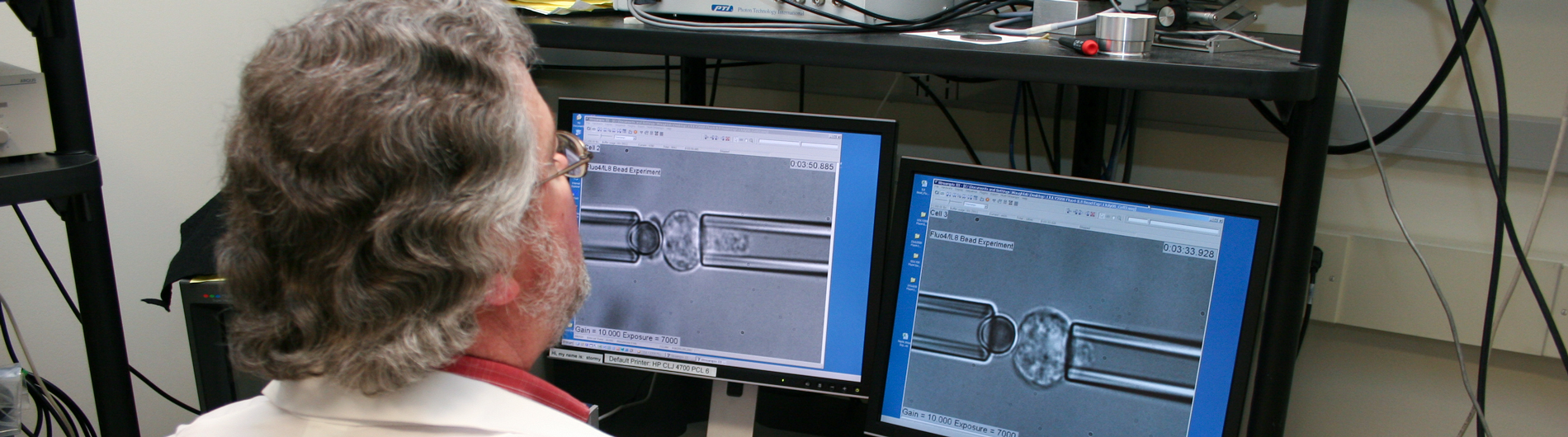
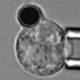
Series of microphotographs showing the micropipette experiment setup.
Most of our work centers on the deformability of blood cells and how blood cell deformability plays a role in health and disease. The two principal directions in our research currently are the role of mechanical forces in the development of red blood cells and how understanding this might help in the production of red cells in vitro, and the role of cell deformability during inflammation, particularly with regard to adhesion between leukocytes (white blood cells) and the endothelium.
Our red cell work includes both basic research into the mechanisms leading to membrane mechanical stability during red cell maturation as well as efforts to develop microfluidic systems to improve the development of red blood cells in culture in the laboratory. Our work in this area involves collaborations with the McGrath lab in Biomedical Engineering, and the Palis lab in Pediatrics. In our basic research, we draw on our expertise on the molecular basis of red blood cell elasticity, and use fluorescence imaged micro-deformation to map the location and failure of cytoskeletal components in maturing red blood cells. In our microfluidic investigations, we use nano- and microfabricated membranes to separate cells of different sizes and deformability and to apply precise constraints on the shape of maturing cells to stimulate them to mature properly.
A red blood cell being sucked into a micropipette.
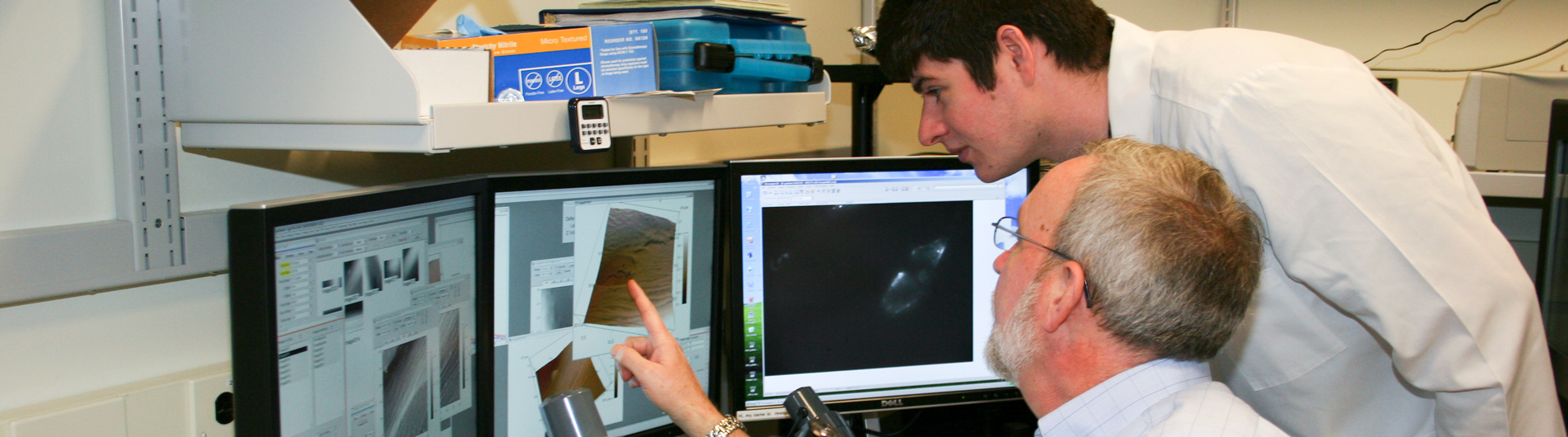
Our work on the importance of cell deformability during inflammation examines the topography and deformability of the surfaces of both leukocytes and endothelial cells to determine how these cell characteristics regulate the ability of cells to adhere to each other. Leukocytes have “wrinkled” surfaces, and many adhesion molecules on the leukocyte surface are confined to the “valleys” between microvilli on the cell surface. The surface topography must be smoothed out, either by mechanical force or by chances in the state of the cell’s cytoskeleton to allow the adhesion molecules to engage with other surfaces and form adhesive bonds. The surface of the endothelium is coated with a carbohydrate-rich layer called the glycocalyx, which can also act as a mechanical barrier to adhesion. We use atomic force microscopy to probe the distribution and mechanical properties of this layer to determine how much of a barrier to adhesion it creates, and how the effectiveness of this barrier changes during inflammation.

Richard E. Waugh, Ph.D.
Principal Investigator
- Dynamic duos: learning to care as a pair in the biparental prairie vole (Microtus ochrogaster).; Frontiers in behavioral neuroscience; Vol 19, pp. 1698616. 2025 Nov 26.
- Characterization of Transient Elasticity in Neutrophils.; Biophysical journal. 2025 Nov 01.
- Shear Conditioning Promotes Microvascular Endothelial Barrier Resilience in a Human BBB-on-a-Chip Model of Systemic Inflammation Leading to Astrogliosis.; Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2025 Aug 27.
- Oxytocin treatment at birth accelerates an epigenetic shift in the oxytocin receptor gene in the maternal brain.; BMC pregnancy and childbirth; Vol 25(1), pp. 777. 2025 Jul 19.
Contact Us
Waugh Lab
University of Rochester
Goergen Hall 237
Rochester, NY 14627