UpToDate Move to Single Sign-on (SSO)
UpToDate Move to Single Sign-on (SSO)
University of Rochester UpToDate Log-in Moving to Single Sign-On (SSO)
UpToDate log in has moved to single sign-on (SSO) using URMC or UR AD credentials. NetID is no longer be accepted as a log in method.
The first time users log in to UpToDate through SSO, a new UpToDate account will be created. This requires ACTION by users who already have an UpToDate Account. Please see information below:
User already has an UpToDate Account
New User/Never had an UpToDate Account
Completed first time SSO registration but did not merge accounts
Merging Accounts Information/Complications/Help
Mobile App & UpToDate Anywhere / Mobile Web Access

User already has an UpToDate Account
Users do NOT need to register for a new UpToDate account if already using an existing account (ie. CME credits or history/bookmarks attached to existing account).
- During the registration process, click the “sign in” link on the registration page to sign in with existing UpToDate account username & password
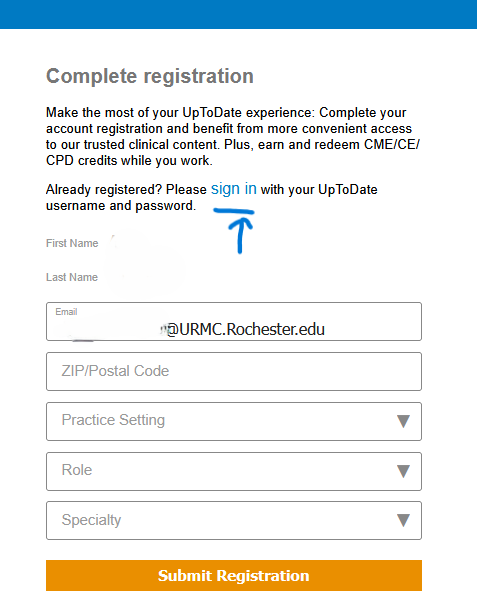
- Completing this step will auto-merge the existing account into a new SSO UpToDate account. This is the PREFERERED METHOD for merging accounts.
- This means the old UpToDate username (assuming it's not URMC or UR e-mail address) will become irrelevant and new username will be URMC or UR e-mail address.
New User/Never had an UpToDate Account
- Complete all the fields on the registration form to create a brand-new SSO account.
Completed first time SSO registration but did not merge accounts
User completed the first time SSO process, a new account was created but user already had an existing account and did not complete the auto-merge step during registration, follow directions to manually merge accounts:
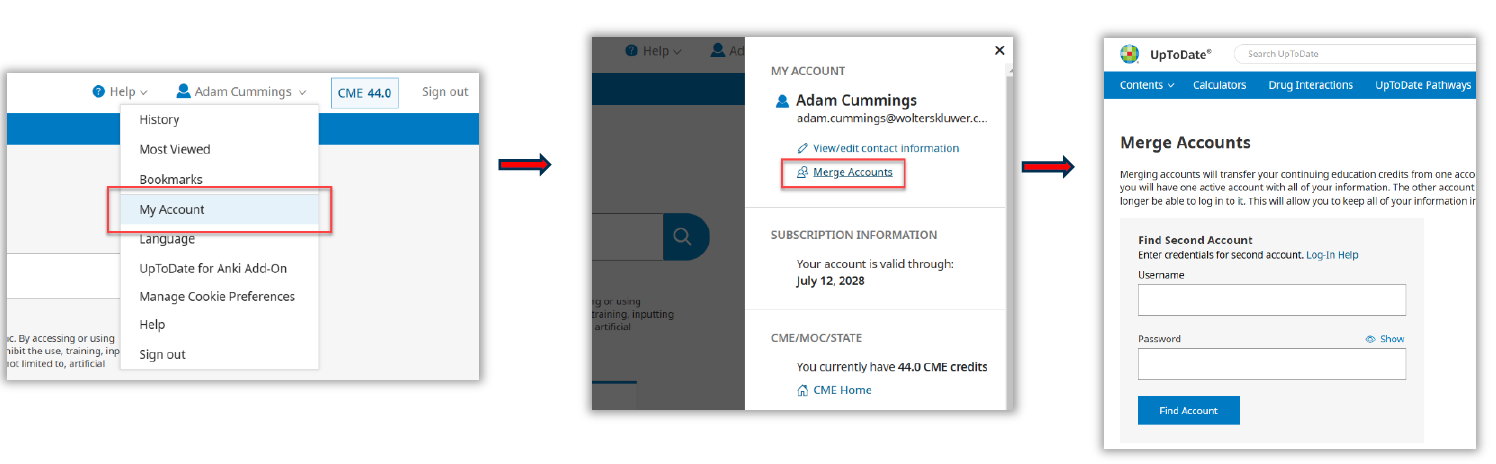
- Log in to UpToDate with SSO
- Click “My Account” and then “Merge Accounts”
- User will be prompted to log in with their original account (using old username/password) to merge accounts.
Merging Accounts Information/Complications/Help
There are certain situations when account merge may not work or produces an error, for example:
- Non-matching last names: If the user’s last name on the two accounts attempting to merge does not match, it will produce an error.
- Please contact UpToDate Customer Service to assist in updating the name on the account, or other merge account questions.
Mobile App & UpToDate Anywhere / Mobile Web Access
After completing one-time registration through SSO:
- Mobile App
- If already using the UpToDate app, log out and log back in with new username (URMC or UR email address), then log in through SSO. Reaffiliating every 90 days is no longer necessary.

- Never used app? Download app through App store. Log in with username (URMC or UR email address) then go through SSO.
- UpToDate Anywhere / Mobile Web Access
- Go directly to UpToDate, enter username (URMC or UR email address) then log in through SSO.
Need assistance? Ask a Librarian