Regulation of Bone Cell Function in Inflammatory Bone Loss
Research Overview
The major focus of the Xing Laboratory is to study the regulation of bone cell function in inflammatory bone loss in diseases such as rheumatoid arthritis. The lab uses combinations of genetically modified animals, in vivo imaging technologies, histology and in vitro cellular and molecular approaches.
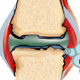
Current NIH research involves the role of TNF alpha in osteoclast-mediated bone loss. TNF plays a critical role in mediating local inflammation and bone erosion in patients with rheumatoid or psoriatic arthritis. It is known to synergize with RANKL to enhance the differentiation of osteoclast precursors (OCPs) to osteoclasts (OCs) in inflamed joints. The lab has shown that TNF and RANKL regulate not only the proliferation of OCPs in the bone marrow, but also their mobilization to the blood and their differentiation and activation in inflamed joints during inflammatory-erosive arthritis.
We are studying the mechanisms that control OCP migration to arthritic joints and the role these cells play in stimulating inflammation in the joint, which we propose mediates a vicious cycle of erosive arthritis. These discoveries could lead to new diagnostics and therapies for the unmet needs of patients that suffer from inflammatory arthritis.
We are also exploring the role of proteasomal regulation in TNF-mediated bone loss. Patients with chronic inflammatory disorders, such as rheumatoid arthritis (RA), often have osteoporosis due to a combination of increased bone resorption and reduced bone formation. Osteoblasts (obls) differentiate from mesenchymal stem cells (MSCs), which are normally controlled by Runx2 and AP-1 transcription factors, whose stability can be regulated by ubiquitination-mediated proteasomal degradation.
Recently, we found that purified MSCs from TNF transgenic (TNF-Tg) mice, a model of RA with severe osteoporosis, had a decreased osteogenic potential and increased ubiquitin E3 ligase WWP1 levels. We are now exploring the role of WWP1 in normal and pathologic bone loss in vivo. Results will enhance our understanding of E3 ligase-mediated protein modification in the regulation of MSC differentiation into osteoblasts under normal and inflammatory conditions.

Lianping Xing, B.Med., Ph.D.
Principal Investigator
- Brain-cervical lymph node crosstalk contributes to brain injury induced by subarachnoid hemorrhage in mice.; Nature communications; Vol 16(1), pp. 8551. 2025 Sep 29.
- Reduced expression of semaphorin 3A in osteoclasts causes lymphatic expansion in a Gorham-Stout disease (GSD) mouse model.; Journal of Zhejiang University. Science. B; Vol 25(1), pp. 38-50. 2024 Jan 15.
- Lymphatic platelet thrombosis limits bone repair by precluding lymphatic transporting DAMPs.; Research square. 2023 Jan 14.
- Distinct mast cell subpopulations within and around lymphatic vessels regulate lymph flow and progression of inflammatory-erosive arthritis in TNF-transgenic mice.; Frontiers in immunology; Vol 14, pp. 1275871. 2023 Jan 14.
Contact Us
Xing Lab
601 Elmwood Ave, Box 626
Rochester, NY 14642