URMC / Labs / Benoit Lab / Projects / Hydrogels for Local Delivery of Therapeutics
Hydrogels for Local Delivery of Therapeutics

Figure 1: Hydrogel biophysical and biochemical characteristics
can be altered to control angiogenic growth factor delivery.
Furthermore, hydrogel chemistry provides avenues for host cell
degradation and subsequent migration into the hydrogel
to form angiogenic networks.
Project Collaborators:
Dr. Hani Awad
Hydrogels can be exploited to encapsulate and deliver cells and biomolecules therapeutically, as delivery characteristics can be intimately controlled through alterations in hydrogel biophysical and biochemical structure. We are interested in using hydrogels to in situ polymerize delivery systems for cells and/or therapeutics that act locally to aid ischemic tissue regeneration, wound healing, or control inflammation.
We hypothesize that hydrogels designed to encapsulate cells and control the availability of cellularly-released paracrine factors and/or other therapeutic molecules such as growth factors, gene vectors, or small interfering RNA, will be useful for these therapeutic strategies. As an example, heparin functionalities can be incorporated into hydrogels to control the release of angiogenic growth factors for treatment of chronic wounds, as depicted in Figure 1.
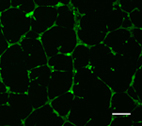
Treatment with
pro-angiogenic peptides
released from poly(ethylene
glycol) hydrogels causes
human umbilical vein endothelial
cells to form honeycomb-like
tube networks.
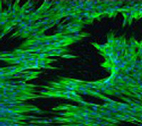
Fibroblasts differentiated
through cell-material inter-
actions have contractile
properties, as indicated by
green staining of α smooth
muscle actin (nuclei in blue.
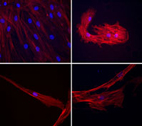
Differentiation can be
monitored morphologically,
as pictured here where
cytoskeleton is stained in red
and nuclei in blue, or through
gene expression analyses.
« back to all projects