URMC / Labs / Benoit Lab / Projects / Targeted Drug Delivery Systems for Musculoskeletal Applications
Targeted Drug Delivery Systems for Musculoskeletal Applications
Project Collaborators:
Dr. Hani Awad, Dr. Edward Puzas
My laboratory has unique expertise in polymeric materials development and has exploited this approach to overcome challenges associated in musculoskeletal tissue-based delivery. These challenges include poor tissue-specific biodistribution and resulting off-target effects. We employ a controlled/living polymerization strategy known as RAFT (reversible-addition fragmentation chain transfer) to synthesize polymers with well-controlled molecular weight, polydispersities, flexible polymer chain end functionalities, and a multitude of architectures, all attributes enabling reproducible, effective, and targeted therapeutic delivery. Our projects in this involve important and key steps in the design of RAFT polymers for potent and targeted delivery of both small molecule drugs and small interfering RNA (siRNA). We are designing drug carriers that have targeted, controlled, and ‘triggered’ material properties – spatially, temporally, and physiologically (e.g., via pH) – to provide drug delivery at the right time, at the right place, and at the right concentrations.
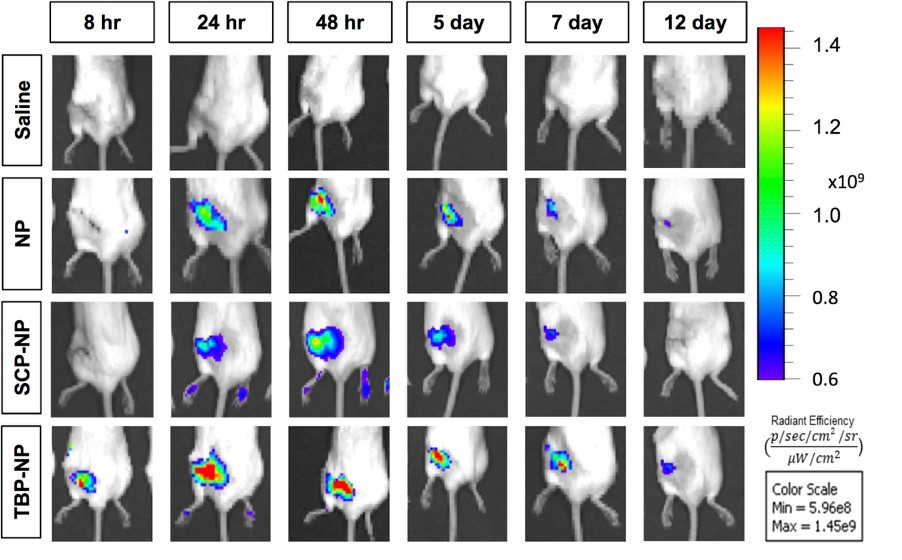
TBP incorporation enhances fracture biodistribution of NP at conventional femur fractures after retroorbital injections (3 days post-fracture, 5 mg/kg NP loaded with 0.42 mg/kg IR780).
One example of our use of targeted drug delivery systems involves development of pharmacologic interventions of non-union fractures. Of the 15 million Americans that suffer from fractures each year, ~10% have impaired healing. Moreover, the incidence of fractures is predicted to increase dramatically as the population ages, motivating the development of effective therapeutic strategies to enhance fracture healing. Impaired fracture healing results from reduced mesenchymal stem cell (MSC) prevalence and differentiation capacity. Several pharmacologic agents have the potential to enhance fracture healing by modulating MSC functions including BMPs, PTH, FGF-2, and Wnt/β-catenin agonists. Of these, BMP-2 and 7 are FDA approved, but only for select non-union fractures due to off-target ectopic bone formation resulted from supra-physiological doses required to achieve desired therapeutic levels at fracture sites. Similarly, many small molecule Wnt/β-catenin agonists have been developed, but they have failed FDA approval due to safety concerns associated with off-target tissue biodistribution. It is clear that a critical technological gap exists in delivery of potent, regenerative drugs to fracture sites while limiting biodistribution to off-target tissues to improve safety and clinical translatability. To address these hurdles, we have developed a fracture-targeted nanoparticle(NP)-based delivery system for Wnt agonists. Targeting is achieved by incorporation of a peptide that binds specifically to tartrate resistant acid phosphatase (TRAP), a protein deposited by osteoclasts in response to bone injury acutely and throughout the healing process. TRAP-binding peptide (TBP) targeted NP exhibit preferential accumulation at conventional femur fractures. Fracture localized activation of β-catenin is greatly increased compared with untreated, free drug, untargeted NP, and scrambled peptide NP controls. Expedited callus formation was observed in fractures treated with TBP-NP-drug versus controls with more rapid ossification of cartilage callus. Finally, the maximum torque to failure of treated fractures was 2.5 to 4-fold greater than controls 4 weeks after treatments. Based on this preliminary data, we hypothesize that this delivery system can be adopted to safely stimulate MSC Wnt/b-catenin signaling leading to healing in a clinically-relevant non-union fracture model. With a long-term goal of treating fracture non-unions using this approach, we propose the following aims: Aim 1: Maximize selectivity of polymer nanoparticle biodistribution to non-union fractures by modulating the presentation of peptide targeting groups. Aim 2: Use dose escalation of TBP-NP-drug to identify doses with maximal Wnt/b-catenin-mediated fracture healing with minimal off-target tissue effects. Aim 3: Assess clinical relevance of non-union fracture healing via delayed treatments with fracture site-specific NPs. Successful completion of these Aims will significantly advance our understanding of how drug delivery systems can be designed to target drugs to bone and augment non-union fracture healing in clinically-relevant models.
« back to all projects