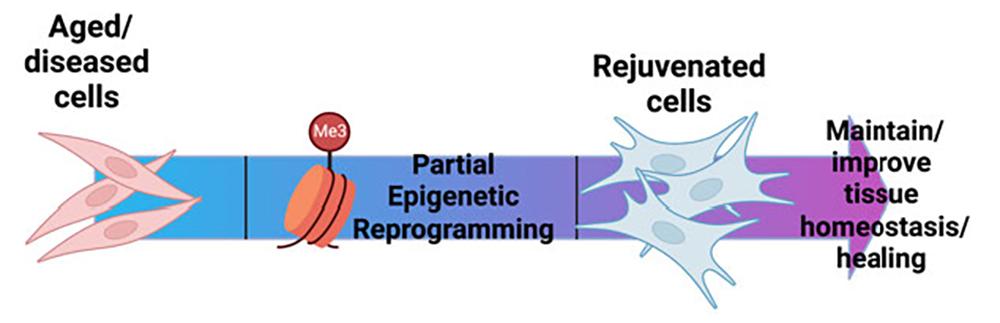
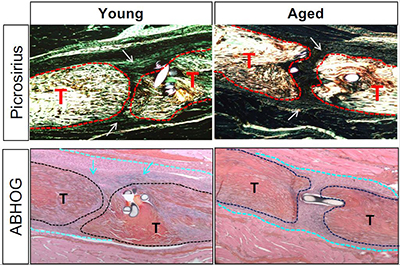
 During aging, tendons demonstrate substantial disruptions in homeostasis, leading to impairments in structure and function. Given the central role of tendon in appropriate skeletal locomotion and ambulation, impaired tendon function contributes to substantial declines in overall function and quality of life during aging. Moreover, aged tendons are more likely to undergo spontaneous rupture, and the healing response following injury is drastically impaired in aged tendons. Thus, there is a clear need to develop strategies to maintain tendon homeostasis and healing capacity through the lifespan. Tendon cell density sharply declines by about 12 months of age in mice, and this low cell density is retained even in geriatric tendons. Our preliminary data suggests that this decline in cellularity initiates a degenerative cascade due to insufficient production of the extracellular matrix components needed to maintain tendon homeostasis. Thus, preventing this decline in tendon cellularity has great potential for maintaining tendon health. In addition, the tenocytes that remain in aged tendon demonstrate substantial alterations in their molecular programs, relative to young tendon cells. Surprisingly, this programmatic skewing does not seem to drive additional homeostatic disruptions, but we hypothesize that it is a key driver of age-related impairments in tendon healing. Thus, reversing this programmatic skewing may restore physiological healing function to aged tendons. While the pathways that drive aging-induced tendon cell death vs. programmatic skewing are likely distinct, epigenetic modifications underly nearly every aspect of cell function. Indeed, partial epigenetic reprogramming has demonstrated tremendous potential in addressing a range of age-related pathologies.
During aging, tendons demonstrate substantial disruptions in homeostasis, leading to impairments in structure and function. Given the central role of tendon in appropriate skeletal locomotion and ambulation, impaired tendon function contributes to substantial declines in overall function and quality of life during aging. Moreover, aged tendons are more likely to undergo spontaneous rupture, and the healing response following injury is drastically impaired in aged tendons. Thus, there is a clear need to develop strategies to maintain tendon homeostasis and healing capacity through the lifespan. Tendon cell density sharply declines by about 12 months of age in mice, and this low cell density is retained even in geriatric tendons. Our preliminary data suggests that this decline in cellularity initiates a degenerative cascade due to insufficient production of the extracellular matrix components needed to maintain tendon homeostasis. Thus, preventing this decline in tendon cellularity has great potential for maintaining tendon health. In addition, the tenocytes that remain in aged tendon demonstrate substantial alterations in their molecular programs, relative to young tendon cells. Surprisingly, this programmatic skewing does not seem to drive additional homeostatic disruptions, but we hypothesize that it is a key driver of age-related impairments in tendon healing. Thus, reversing this programmatic skewing may restore physiological healing function to aged tendons. While the pathways that drive aging-induced tendon cell death vs. programmatic skewing are likely distinct, epigenetic modifications underly nearly every aspect of cell function. Indeed, partial epigenetic reprogramming has demonstrated tremendous potential in addressing a range of age-related pathologies.
To address this, we will define the multi-scale mechanisms of age-related tendon degeneration using a combination of genomics, histological, and mechanical analyses. We will then determine the efficacy of partial reprogramming to maintain tendon structure-function through the lifespan.
We will also define how aging alters the cellular response to tendon injury using a well-established model of tendon healing. We will then demonstrate that partial reprogramming can successfully restore the tenocyte functional plasticity that is required for physiological healing. Successful completion of these studies will define the tendon aging signature and establish partial reprogramming as a novel approach to maintain tendon health and healing capacity through the lifespan.