Research
Molecular and Signaling Mechanisms of Synaptic Plasticity in Memory Formation and Mental Health
Synapse has been shown to undergo persistent modifications in response to different patterns of activity which is termed synaptic plasticity, with long term potentiation (LTP) and long term depression (LTD) being its major forms. Synaptic plasticity has been hypothesized to underlie the experience-dependent brain functions, such as learning, memory and anxiety. We are interested in how NMDA receptors function in inducing these many forms of synaptic plasticity. We are not only interested in short term modifications in the synaptic protein composition through calcium mediated signaling pathway, but also CREB mediated gene transcription which provides new proteins for long term modification of the synapse.
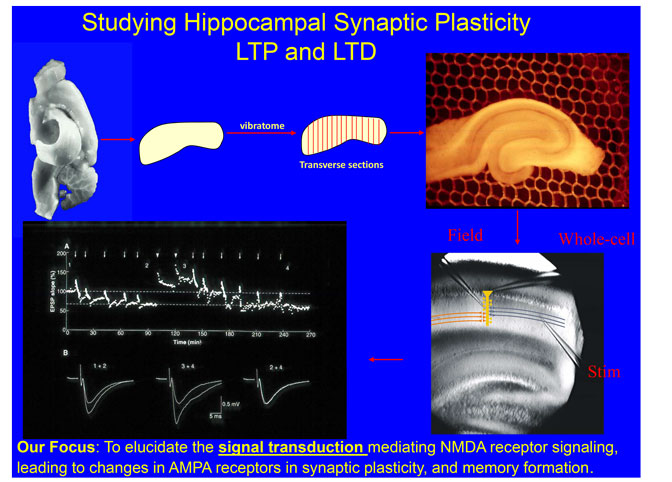
We study the signaling roles of protein phosphatase-1 (PP1), mainly focused on its inhibitors (inhibitor-1 and inhibitor-2 (I-2)) and targeting proteins (neurabin, spinophilin, HDAC family molecules and nuclear inhibitor of PP1 (NIPP1)), in synaptic transmission, plasticity, CREB mediated gene transcription and aforementioned brain functions. We used a variety of techniques, including confocal immuno-fluorescence, immuno-gold electron micrograph (EM), biochemistry (including IP, western blotting), viral mediated gene transfer (including lenti-virus mediated knocking down genes of interest and then replacing it with recombinant proteins---molecular replacement), FRET/BRET imaging, transgenic/knockout mice (I-2, NIPP1 and PP1), cellular electrophysiology and behavioral testing. We use primary hippocampal/cortical cultures, acute slices and knockout mice for our proposed studies.
Our studies suggest that neurabin is the major PP1 synaptic scaffolding protein regulating the induction threshold of LTP vs LTD. Moreover, we found that I-2 is a novel memory suppressor. Furthermore, we found that I-2 positively regulates PP1 function in vivo in memory formation, a paradigm-shift discovery which is in stark contrast to I-2’s known in vitro function of inhibiting PP1 activity. We are currently testing the hypothesis that I-2 positively regulates PP1 function by increasing the binding between PP1 and its anchoring proteins. Additional projects include:
- Elucidating the signaling mechanisms underlying differential regulation of I-2 phosphorylation by NMDA receptors and L-type calcium channels
- Structure function study of I-2 in homeostatic synaptic plasticity as well as Hebbian synaptic plasticity
- Determining the role, and regulation by NMDA receptor signaling, of I-2-PP1 complex in escalated anxiety in alcohol dependent rats
- Determining the role of NIPP1 in synaptic formation, plasticity and memory formation
- Developing small molecule inhibitor of I-2 which potentially could be used in future for treating memory loss in patients
- Studying the effect of environmental toxins such as arsenic on PP1 expression and memory impairment
- Testing the hypothesis that the de novo mutation found in PP1 gene in human patients with intellectual disability is responsible for deficits in synaptic plasticity and memory formation in a rodent model.
- Determine the signaling mechanism of amygdala I-2 in promoting anxiety associated with alcohol withdrawal
- Using optogenetic technique to determine the role of I-2 in different brain regions in anxiety, memory formation and other experience dependent brain functions.