About Us

CNL-R is a group of scientists, clinicians, and technicians based in the University of Rochester Medical Center, Department of Neuroscience. As a group we are interested in understanding how the brain processes input from our sense organs. We have a particular interest in how conditions and disorders with genetic and neurological components affect sensory perception and sense-related cognition. To date, researchers in our lab have published articles related to Autism Spectrum Disorder, Dyslexia, Multiple Sclerosis, Multisensory Integration, ADHD, and Rett Syndrome, to name a few. Current research foci include multisensory integration, dyslexia, Batten Disease, Rett Syndrome, autism, neurological complications of AIDS, and movement disorders in aging.
We utilize a variety of techniques including electroencephalography, electrocorticography, functional magnetic resonance imaging, diffusion tensor imaging, 3D motion capture, and transgenic murine models to study the characteristics of the normal and abnormal brain. Our mission is to identify and understand the physiology and fundamental deficits behind specific and debilitating diseases, syndromes, and disorders, and to connect these deficiencies to common genetic, physiological, and behavioral traits. Furthering our knowledge of these diseases will allow researchers to motivate therapeutic interventions and definitively measure potential therapeutic benefits.
The Lab is located in the Rochester Center for Brain Imaging and can be contacted via cogneurolabrochester@gmail.com or (585) 275-1674.
If you are interested in participating as a subject in any of our studies, please see the listings in the Help Wanted panel.
Lab Methodologies
Electroencephalography – EEG
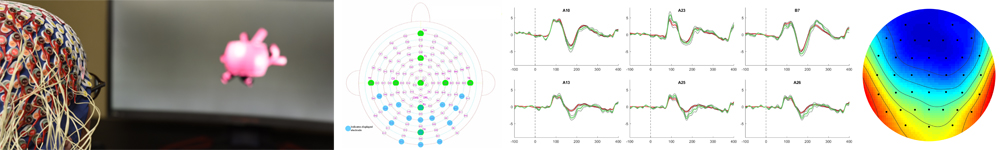
Electroencephalography (EEG) is a classical neuroimaging technique that allows researchers to record electrical potentials generated by groups of neurons in the brain, non-invasively, using harmless electrodes placed on the scalp surface. Clinical use of the EEG began in the mid 1900’s for diagnosing major brain disorders like epilepsy. Over the past 50 years researchers have uncovered an unbelievable wealth of information in the EEG signal relating to the ongoing cognitive processes in the brain, to the extent that – using only the voltages recorded at the scalp surface – one can know whether a research subject is listening to spoken words, viewing images of faces, or being presented with familiar vs. unfamiliar images. Ongoing studies using EEG seek to further uncover and characterize scalp recorded potentials that relay information about how the brain manages its incomprehensible flow of information. EEG recordings represent a foundational neuroimaging technique in the CNL-R.
Magnetic Resonance Imaging – MRI
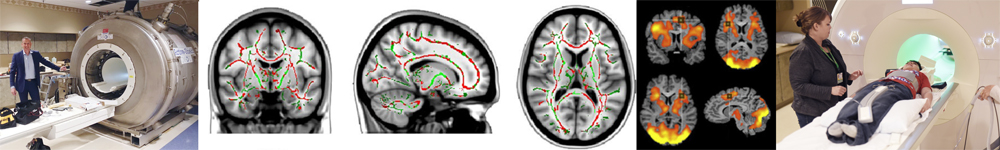
Magnetic Resonance Imaging (MRI) was developed in the 1970’s and allows for the formation of pictures of internal anatomy without the use of radiation. MRI’s are used extensively in the clinical setting to identify abnormal anatomy or localize internal trauma. In the field of neuroscience research, MRIs have provided an exceptional method by which to correlate subtle differences in brain organization and anatomy to behavioral, psychological, and cognitive deficits or abnormalities. Furthermore, a special adaptation of the MRI technique allows for the collection of so called “functional” MRIs (fMRI), whereby a direct correlate of brain-cell activity can be measured over time and one can thus demonstrate the spatial and temporal extents of ongoing cognition within brain structures.
Another adaptation of the MRI technique provides what is known as tractography data, a method known as Diffusion Tensor Imaging (DTI). The use of DTI allows for an assessment of the integrity of the brains fiber bundles, or white matter tracts, which serve to pass information from one brain region to another and are thus critically important to the brains functioning. Ongoing research aims to describe the extent to which various diseases or disorders may be the result of, or contribute to, differences in white matter integrity, and functional or structural abnormalities. The CNL-R is located in the Rochester Center for Brain Imaging, giving lab members access to a research-dedicated 3 tesla MRI scanner.
Behavioral Phenotyping
Behavioral phenotyping generally refers to the administration of standardized and thoroughly validated question or activity batteries meant to quantify the extent or degree of severity of various neurological, psychological, or behavioral conditions and disorders. These batteries, such as the SKID, PERSNAPS, FLUBBER, or BLEKS can then be correlated to other neuroimaging findings to provide insights to whether neuroimaging data correlates with the severity of the disorder, or which brain areas may be involved. Ongoing collaborations between the CNL-R and departments of Psychiatry and Neurology allow for lab members to be trained by clinical experts in the administration of these assessments.
Electrocorticography - ECoG
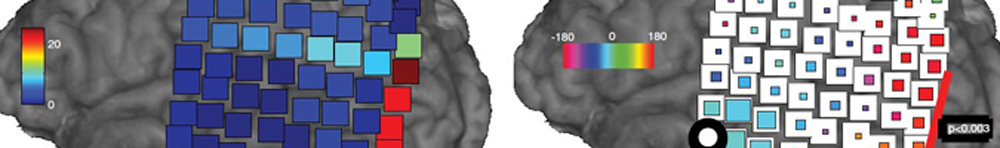
Electrocorticography (ECoG) is a neuroimaging technique that places specially designed electrodes directly on the surface of the brain. These electrodes serve to record the microvoltage potentials generated by populations of discharging neurons. Because the dura mater, skull, and scalp are not opposed between the recording electrodes and discharging neurons, a much more precise spatial localization of the voltage sources is possible, compared to standard EEG recordings. The spatial sensitivity of the ECoG paired with the high temporal resolution of electrical recordings provides a powerful amount of information to researchers investigating the brains convoluted landscape. Because of the highly invasive nature of placing ECoG electrodes, studies using this technique rely on clinical patient populations who are having electrodes implanted for treatment purposes. An ongoing collaboration with URMC’s Epilepsy and Neurological Surgery departments makes this incredibly valuable research possible.
Mobile Brain Body Imaging – MOBI
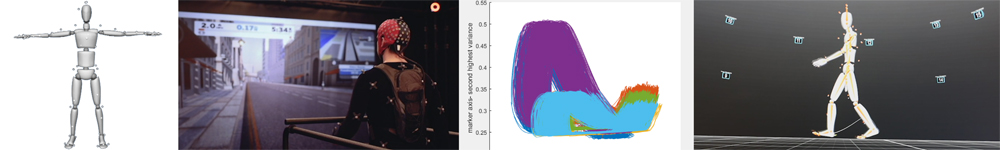
3 Dimensional Motion Capture is used extensively in Hollywood and in the video game industry to create the fluid movements in computer assisted graphics that we are now accustomed to seeing. Bringing this technology into the laboratory setting allows neuroscientists to track, with incredible temporal and spatial resolution, the subtle aspects and perturbations to our stereotyped body movements that result from changes in cognitive load, or declining motor performance with age. The CNL-R has recently upgraded to a state of the art motion capture system that allows for high-resolution tracking of thousands of points, simultaneously, in a 3000 cubic foot volume. The implementation of motion tracking in neuroscience research greatly expands our experimental possibilities as body movements, a set of variables that were previously impossible to track reliably, can now be embraced as we begin to untangle the true breadth and depth of the perception action cycle.