URMC / Labs / Goldman Lab / Major Lines of Investigation / Inducing Endogenous Progenitors as a Means of Treatment in Huntington’s Disease
Inducing Endogenous Progenitors as a Means of Treatment in Huntington’s Disease
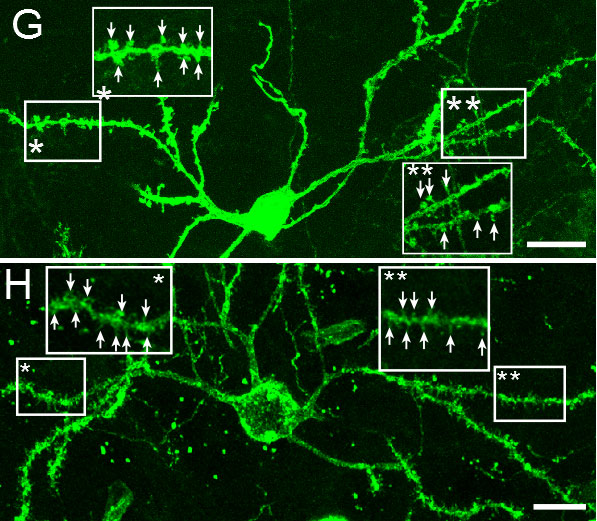
New medium spiny neurons integrating into the brain’s existing striatopallidal circuitry.
Besides my group’s efforts in glial progenitor cell biology and use, we have also a longstanding interest in adult neurogenesis and its potential induction for therapeutic purposes. In the course of my group’s longstanding work on adult neurogenesis in the songbird brain – a finding that I made as a graduate student many years ago and continue to investigate to the present day – we learned that two factors, the neurotrophic differentiation agent BDNF and the gliogenic suppressor noggin, are critical to permitting ongoing neuronal addition to adult brain tissue. On that basis, we asked whether the forced over-expression of these agents in the forebrain subependyma, the region that harbors persistent neural stem cells, would be sufficient to direct neuronal addition to otherwise non-neurogenic regions of the adult mammalian brain. We found that this was indeed the case, and in a series of papers over the past decade, established BDNF and noggin over-expression as a feasible strategy by which to induce neuronal addition to the adult murine neostriatum.
Huntington's disease is a neurodegenerative disease characterized in part by the loss of neostriatal neurons, and in particularly of medium spiny striatopallidal projection neurons, the same phenotype recruited in response to BDNF and noggin. On that basis, we found that intracerebroventricular injection of adenoviral BDNF and noggin is indeed sufficient to not only trigger the addition of new neurons to the neostriatum, but also to slow disease progression in R6/2 mutant huntingtin mice. More recently, we used intraventricular delivery of adeno-associated virus (AAV4), an ependymotrophic vector that allows persistent transgene expression, to sustain both BDNF and noggin expression and the recruitment of new medium spiny neurons in both wild-type and R6/2 mice. Using R6/2 x nestin-CreERT2 x EYFP mice treated with either intra-ventricular AAV4-BDNF/noggin, we proved the origin of these new cells from nestin+ subependymal progenitors, while pallidal backfills followed by patch clamp confirmed the maturation and circuit integration of these new neurons. Most importantly, the AAV4-BDNF/noggin-treated R6/2 mice exhibited delayed motor deterioration and substantially increased survival. To validate the potential relevance of these observations to humans, we gave ICV injections of adenoviral BDNF and noggin to normal squirrel monkeys, in which we similarly observed significant treatment-associated striatal neuronal addition.
These observations suggest the potential feasibility of induced neurogenesis as a promising means of disease modification in HD. Going forward, they suggest the strong need and likely benefit of identifying necessary and sufficient signals for inducing neuronal differentiation from resident progenitor cells, and for regulating their adult parenchymal migration and circuit integration. In addition, these observations suggest the need for new genetic targets and gene therapeutic strategies for inducing and supporting neuronal addition to the brain from resident progenitor cells. We have initiated these studies by profiling human medium spiny neuron progenitors, and by focusing on the receptors expressed by these cells, have predicted the ligands by which they may be supported and their differentiation influenced. Using this information, we hope to design an increasingly more efficient and targeted strategy for the robust induction of neural addition to diseased or injured neural circuits throughout the brain, and not just those of the neostriatum. This rational search for new initiators and regulators of adult neuronal addition to extrastriatal brain regions will be focus of effort for years to come, as will be the optimization of adult neuronal addition strategies in those regions for which we already have effective induction protocols.
« back to all projects