Biofilm Methods for Scanning Electron Microscopy (SEM)
The specimens coated with biofilm are fixed in a 0.1M Millonig’s buffered 2.5% glutaraldehyde/4.0% paraformaldehyde overnight at 4°C, rinsed in two changes of Millonig’s buffer (15 minutes each), post-fixed 45 minutes in buffered 1.0% osmium tetroxide, dehydrated at 30 minute intervals in a graded series (50 to 95%) and finally in 100% ethanol. The specimens are critical point dried or transitioned at 30 minute intervals into solutions of 100% ethanol mixed with Hexamethyldisilizane (1:1, 1:2, 1:3) ending with four changes (one hour each) of 100% HMDS with the final change allowed to evaporate overnight in a fume hood. The dried samples are then mounted onto carbon sticky tape on top of aluminum stubs and sputter coated (Denton sputter coater) with gold for 90 seconds. Digital images were taken with a Gatan imaging system on a Zeiss Auriga Supra Field Emission SEM.
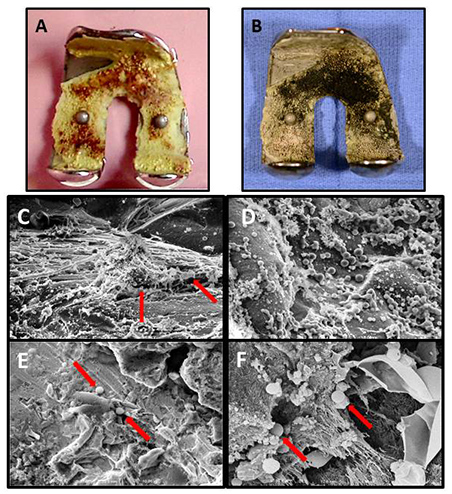
(A, B) An example of femoral hardware removed from a patient's infected knee placed into EM fixative in the operating room and then processed for scanning electron microscopy (SEM). (C-F) Examples of SEM micrographs from the implant above and others:
- Pale yellow cement on implant's inverse side displaying red-brown biofilm.
- Same implant after 1.0% osmium tetroxide staining (now black) showing the extent of the patient's biofilm covering the cement of the implant.
- From the above implant (B) fibrin cables and S. aureus (arrows) x 3,000;
- Colonies of S. aureus covering the surface of the implant in (B) x 5,000;
- Cocci on the cement surface from a fibular implant (arrows) x 3,000;
- High magnification image from a patella implant also displaying fibrin used as a scaffold for several S. aureus cocci forming biofilm (arrows) x 10,000.