Welcome to the Small Lab
The overall goal of the Small lab is to better understand the mechanisms that control cell identity and lineage commitment by studying the transcriptional regulation and function of cardiac tissue-restricted genes. Our motivation is to decipher how disruption of cardiac gene expression programs in heart disease contributes to cellular pathophysiology and the decline in cardiac function. There are two major themes of research within the lab that we study using mouse genetics, cell biology, advanced imaging, bioinformatic and biochemical approaches:
- Cardiac fibroblast plasticity and the development of cardiac fibrosis. The transition to heart failure following cardiac insult is the result of irreversible cardiomyocyte loss and the development of cardiac fibrosis, which impedes contractility and can initiate lethal arrhythmias. Cardiac fibrosis arises from the aberrant and persistent stimulation of fibroblasts, the main source of extracellular matrix in the heart, in a pathological attempt to repair damaged tissue. We utilize gene expression profiling in animal models of heart disease and human heart failure patient samples to identify novel regulators of cardiac fibroblast accumulation and myofibroblast activation in health and disease. We also utilize high-throughput screening and pre-clinical animal studies to develop novel pharmacological and gene-targeting strategies to block or reverse cardiac scarring and the progression of heart failure.
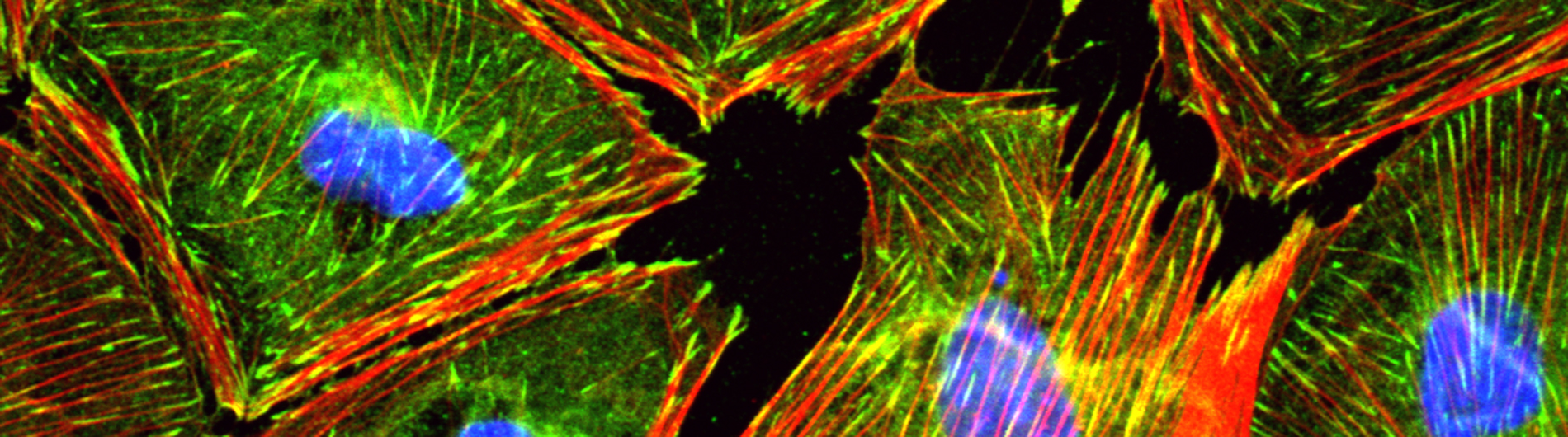
- Epicardium-derived progenitor cell mobilization. Epicardium-derived progenitor cells (EPDCs) can differentiate into cardiac fibroblasts and coronary blood vessels, and secrete signals that stimulate cardiac growth during embryonic development. We are striving to understand how EPDCs differentiate into the appropriate cell type based upon their location within the heart. We have identified a mechanosensitive gene program that is essential for EPDC migration and subsequent differentiation into fibroblasts and perivascular cells. Ongoing studies are aimed at evaluating whether disruption of mechanosensitive transcriptional programs within the epicardium might contribute to cardiomyopathy. Our long-term goal is to harness the regenerative potential of the epicardium to improve cardiac repair.
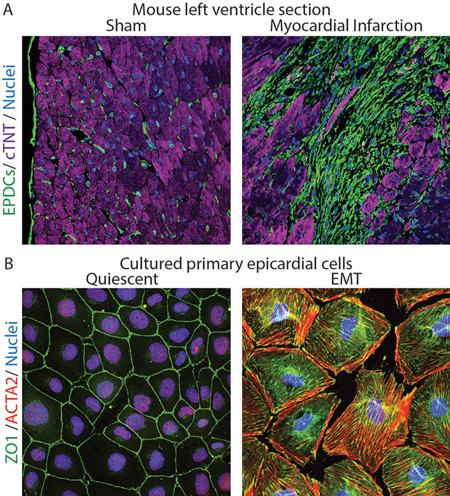
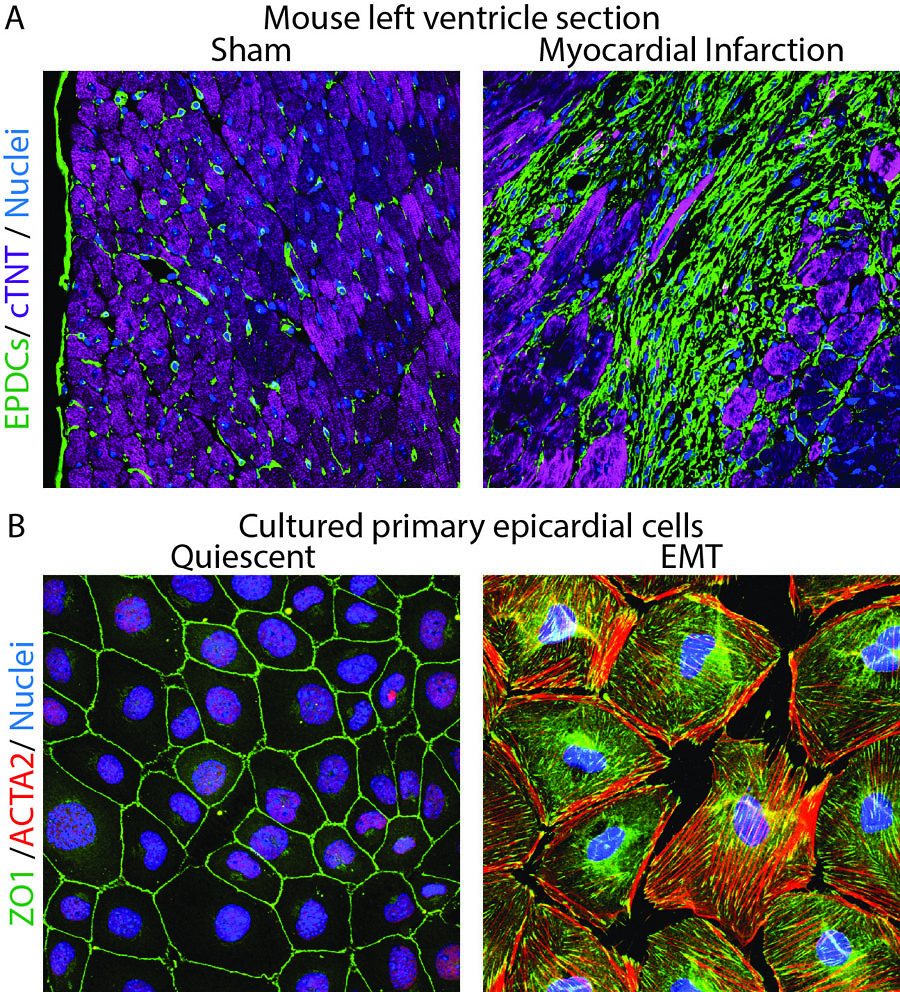
Figure Legend
A. Histological sections from the left ventricle of mice subjected to sham surgery or myocardial infarction were immunostained with antibodies to visualize GFP-positive fibroblasts (green), cTNT-positive cardiomyocytes (purple) and DAPI-positive nuclei (blue). Note expansion of fibroblasts in ischemic myocardium.
B. Cultured epicardial cells were immunostained with antibodies to visualize ZO1 positive cell adhesions (green), ACTA2-positive stress fibers (red) and DAPI-positive nuclei (blue). Note the tightly packed cobble-stoned morphology of epicardial cells in quiescent cultures and the loss of adhesions and accumulation of robust stress fibers in epicardial cells being mobilized to undergo epicardium-to-mesenchymal transition.

Eric M. Small, Ph.D.
Principal Investigator
Publications
View All Publications- Slit2-robo signaling regulates angiogenesis and repair following myocardial infarction.; Journal of molecular and cellular cardiology; Vol 210, pp. 28-42. 2025 Nov 05.
- Epicardial contributions to fibro-inflammatory signaling in a Pkp2-deficient arrhythmogenic cardiomyopathy model.; bioRxiv : the preprint server for biology. 2025 Oct 09.
- Deletion of RSK2 kinase alleviates age-dependent hypertension.; bioRxiv : the preprint server for biology. 2025 Mar 14.
- Whole genome transcriptomics reveal distinct atrial versus ventricular responses to neonatal hyperoxia.; American journal of physiology. Heart and circulatory physiology. 2025 Mar 06.
- P53 Regulates the Extent of Fibroblast Proliferation and Fibrosis in Left Ventricle Pressure Overload.; Circulation research. 2023 Jul 06.
- Cardiac pericytes mediate the remodeling response to myocardial infarction.; The Journal of clinical investigation; Vol 133(10). 2023 May 15.
Affiliations
- Medicine
- Biomedical Engineering
- Pharmacology & Physiology
- Aab Cardiovascular Research Institute
- NIH T32 Training Grant in Cellular, Biochemical & Molecular Sciences
- NIH T32 Training Grant in Immunology
- Pulmonary T32 Training Grant
- Biomedical Genetics and Genomics Ph.D. Program
- Cellular and Molecular Pharmacology and Physiology Ph.D. Program
- Master’s Degree in Physiology
- PhD Program in Pathology - Cell Biology of Disease
Lab Photos
Contact Us
Small Lab
601 Elmwood Ave
Box CVRI
Rochester, NY 14642