Innovation in Advanced Technologies
 What is CLEOPE?
What is CLEOPE?
The mission of the CENTER FOR CLINICAL EVALUATION OF EMERGING POINT-OF-CARE TECHNOLOGIES (CLEOPE) is to facilitate the validation of novel point-of-care monitoring and diagnostic technologies for clinical practices by integrating medical, technical, clinical, and economic resources and expertise into a comprehensive set of services to academic and industry stakeholders.
Why CLEOPE?
Significant growth in elderly populations, coupled with a rising prevalence of chronic diseases, has caused the healthcare systems in the U.S., Europe, and Asia to undergo a strategic change in focus by moving from diagnosis to prevention for various chronic disease states. This, in turn, has stimulated the growth and development of technologies for home care, remote monitoring, telehealth, and self-monitoring. Devices worn on or close to the body are expected to produce the most ground-breaking of these innovations. These “Emerging Health Technologies” lower the usability constraints and increase the monitoring frequency and duration, facilitate diagnostic testing; hence, delivering new insights into disease progression and patient status. As an example, the market for wearable technologies is predicted to reach up to $53 billion worldwide, with 25-35 percent annual growth within the next three years.
CLEOPE is designed to help organizations to streamline the validation of eHealth technologies by facilitating access to resources such as: clinical study design, statistics, patients access, clinical experience, support for submission to Federal funding programs, a large portfolio of scientific experts and researchers.
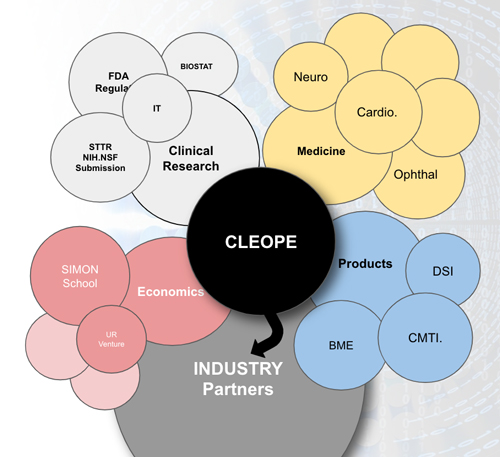
CLEOPE is Organized into Four Components
Enabling your company to get STTR/SBIR awards to fund your R&D programs
The novel POC technologies also referred as Point-of-Care technologies have gained a significant momentum in federal agencies and international scientific societies such as the NIH and the IEEE society which combined forces to facilitate translational research around point of care.
The CCRC has been intervening in such National meeting to share their experience in developing these technologies under federal funding including R01, STTR and SBIR-partnership programs. During these interactions, the stakeholders from the academia and industry commonly agreed on the need to develop a tool facilitating the access to clinical resources needed for the validation/verification of POCT. Interestingly, this path has not been uniformly formalized and current stakeholders relies on multiple and expensive collaborative efforts to assemble all required components.
CLEOPE is designed to facilitate the development of such collaboration. by leveraging the multiple scientific and logistic resources of the University of Rochester.
Accessing unique sets of ECG for validation and development of your technologies: Access to the largest open database of fully-digital ECG in the world
The objective of the Center and its Telemetric and Holter ECG Warehouse (THEW) is to provide access to continuous electrocardiographic data to for-profit and not-for-profit organizations for the design and validation of analytic methods to advance the field of quantitative electrocardiography with a strong focus on cardiac safety. The THEW has been used by 70 Universities and 25 companies worldwide.
Delivering unique resources for conducting Clinical trial for the validation of innovative technologies
The CLEOPE provides access to unique resources required to conduct clinical trial that includes access to patients with a broad spectrum of diseases. The CLEOPE is part of the University of Rochester Medical Center and therefore benefits from a large network of clinical and Medical departments and their expertises. Also, CLEOPE is hosted by the CCRC and the Clinical and Translational Science Institute, both organizations have a long and unique set of clinical research experiences to design and conduct clinical studies and trial of any size for drug and device safety and efficacy assessment. CLEOPE enable access to these resources to stakeholders outside fo the University fo Rochester through the CLEOPE initiative. If your organization is interested in learning more about collaboration please contact Jean-Philippe Couderc, PhD.
Facilitating access to FDA clearance and regulated markets
FDA clearance for medical device is a long and tedious process that requires experience in interacting with the FDA and the overall process of FDA submission. The CLEOPE team has been working with the CDRH at the FDA for various research projects and help industry player to make sure the data produced in their validation study provide the set of information fitting to their technology intended use hence facilitating the submission to the FDA.
As a startup, non-dilutive grant funding can be invaluable. Even for seasoned NIH veterans, this process is difficult and a collaboration with UR for submitting NIH grants that incorporate clinical testing have been equally invaluable to my company. The UR team is a pleasure to work with and their expertise with clinical trials significantly increases the strength of any grant application." Nicholas Conn, CEO, Heart Health Intelligence, HHI.