URMC / Labs / Benoit Lab / Projects / Radioprotection and Regeneration of Salivary Glands
Radioprotection and Regeneration of Salivary Glands
Project Collaborator:
Dr. Catherine Ovitt, Lisa DeLouise, Melinda Larsen (University of Albany), Paul Dunman
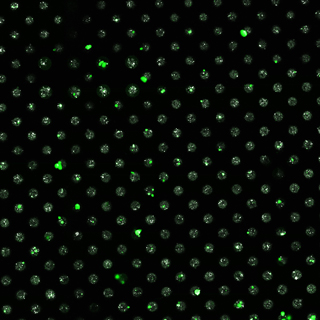
Salivary gland cells seeded within MB chips show promising survival (green fluorescence) as well as assembly into tissue-like structures.
For more than 50,000 patients a year in the U.S. diagnosed with head and neck cancers, severe loss of salivary gland function is an unavoidable outcome of radiation therapy used in treatment. A direct way to prevent xerostomia is to protect the salivary gland cells from radiation damage. Thus, we developed a radioprotective strategy using siRNA-nanoparticle complexes to block genes associated with radiation damage. This strategy results in a remarkable preservation of the histology and function in the treated salivary glands and current work is focused on the long-term safety and efficacy of this rescue. Additionally, to enable the study of salivary gland cells and identify materials transplantation approaches to enable salivary gland structural and functional rescue, we are developing hydrogel microenvironments. We have identified environmental and cellular cues required for salivary gland cell survival, proliferation, and apicobasal organization. The hydrogels are currently being used to transplant cells into regenerating atrophic glands and damaged irradiated glands as a regenerative approach.
Furthermore, efforts to study radiosensitivity to discover effective radioprotective and regenerative strategies have been hampered by the inability to culture salivary gland mimetics in vitro, due to loss of secretory acinar cell phenotype. Thus, we are also developing functional human salivary gland tissue. Our labs have pioneered the use of hydrogel encapsulation to culture salivary gland cells in vitro. While encapsulated salivary gland cells organize into structures with apicobasal polarity and expression of secretory acinar markers, the macroscale nature of hydrogels precludes their high-throughput use. Thus, we will utilize our microbubble (MB) array technology as a high-throughput, modular platform for the tissue chips. MBs are micron-scale spherical cavities molded in polydimethylsiloxane (PDMS). MBs have the advantage of length scales and curvatures similar to the secretory acinar unit of glands, providing a niche that promotes cell-cell contact and the concentration autocrine and paracrine factors that have been shown to enhance tissue assembly. Successful development of salivary tissue chips will be transformative; by enabling in vitro analysis of functional gland mimetics, our ability to pursue therapeutic strategies, radioprotective and regenerative, will be dramatically improved.
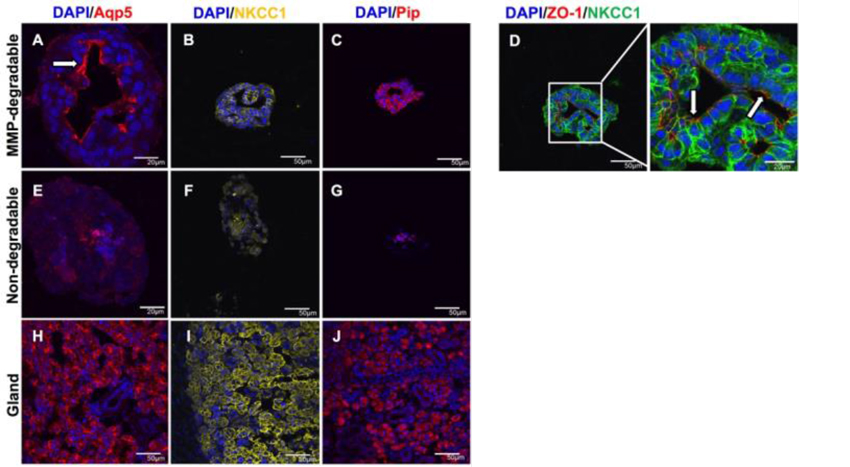
MMP-degradable hydrogels promote lumen-like structures, apicobasal organization, and secretory marker expression. Immunohistochemistry was used to analyze Aqp5 (red; A,E,H), NKCC1 (yellow; B,F,I), Pip (red; C,G,J), and co-stained ZO-1 (red) and NKCC1 (green) (D) in SGCs encapsulated in MMP-degradable hydrogels (A-D) and non-degradable hydrogels (E-G) at day 14. The submandibular gland is shown for reference (H-J). Arrows indicate apical localization. DAPI counterstain was used to visualize nuclei (blue). Scale bars = 20 µm or 50 µm25.
« back to all projects