News
20242023202220212020
Sayeeda Zain fall travel award winners
Thursday, December 15, 2022
We are pleased to announce the winners of the Sayeeda Zain Fall Travel awards: Gabrielle Kosoy, Franziska Stegemann, Jennifer Twardowski and Adrian Molina Vargas.
The Sayeeda Zain Travel Award honors the distinguished career and charitable life of Dr. Sayeeda Zain. The award is given in the fall and spring semesters in recognition of research excellence to support travel and related expenses for attendance at a scientific conference or corporate internship to gain practical experience. The next round of Sayeeda Zain Travel Awards will be offered in Spring 2023.
Thank you to all those who applied and congratulations to Gabrielle, Franziska, Jennifer and Adrian!




Biochemistry and Biophysics Department Student Attends Cold Spring Harbor Laboratory cryo Electron Microscopy Course
Monday, November 28, 2022
Congratulations to PhD candidate Christopher Kirchhoff for attending the competitive, two-week course on cryo electron microscopy (cryoEM) at the Cold Spring Harbor Laboratory (CSHL). The goal of the CSHL course is to equip young investigators with the tools to determine cryoEM structures with rigor and reproducibility.
Chris will use his CSHL training to determine cryoEM structures of splicing factors in the Kielkopf lab.

Dr. Kevin Campbell to present the inaugural Paul LaCelle Lecture November 2, 2022
Monday, November 7, 2022

Professors Peter LaCelle, Kevin Campbell, and Kristina LaCelle-Peterson
On Nov. 2, Dr. Kevin Campbell, from the Department of Molecular Physiology and Biophysics of the University of Iowa presented the inaugural lecture in a newly-endowed annual lecture series honoring the memory of Paul LaCelle, M.D. The lecture, entitled “Mechanistic Insights and Therapeutic Approaches for Matriglycan-Deficient Muscular Dystrophy,” described Dr. Campbell’s research into fundamental biochemical aspects of muscle physiology, as well as applications of his work to understanding and treating muscle diseases. Dr. Campbell received his Ph.D. degree in Biophysics from the University of Rochester in 1979. He is currently a Howard Hughes Investigator, chair of the Department of Molecular Physiology and Biophysics, and director of the Wellstone Muscular Dystrophy Specialized Research Center at the University of Iowa. Dr. Campbell is the author of nearly 500 scientific publications and has received many honors and awards including membership in the National Academy of Sciences.

Professors Mark Dumont, Kevin Campbell, and John Lueck
The LaCelle lecture series, which is expected to be an annual event, was initiated to honor the memory of Dr. Paul LaCelle, a collegial and esteemed member of the URMC faculty for 48 years. Dr. LaCelle, who received his M.D. degree from the University of Rochester in 1959, served as chair of the Department of Biophysics for 19 years and as Senior Associate Dean for Graduate Studies for 12 years until his retirement in 2008. The Department of Biochemistry and Biophysics was pleased that the inaugural LaCelle lecture was be attended by Dr. LaCelle’s son, Dr. Peter LaCelle, Professor of Biology at Roberts Wesleyan University, and his daughter, Dr. Kristina LaCelle Peterson, Professor of Religion at Houghton College.
Passing of Emeritus Professor George Kimmich, PhD
Tuesday, October 25, 2022
 George Kimmich, PhD Emeritus Professor and former Professor in Biochemistry and Biophysics passed away October 8th, 2022 at the age of 80. Funeral services were conducted Tuesday October 18, 2022 at the Edgewood Church, 111 East Jefferson Road, Pittsford, New York. An obituary can be found here:
George Kimmich, PhD Emeritus Professor and former Professor in Biochemistry and Biophysics passed away October 8th, 2022 at the age of 80. Funeral services were conducted Tuesday October 18, 2022 at the Edgewood Church, 111 East Jefferson Road, Pittsford, New York. An obituary can be found here:
https://www.swartzfuneralhomeinc.com/obituary/george-kimmich.
George first joined the university as an Assistant Professor in the Biophysics Department in July 1970, and was appointed to the rank of Professor in 1983. He led an active research program for decades with interests in the biophysics of membrane ion channels and metabolite co-transporters, publishing many landmark papers, mostly in collaboration with his long-standing technician, Joan Randles. He served as Editor-in-Chief for the journals Membrane Biochemistry and The American Journal of Cell Physiology, and was a member of numerous study sections and review panels. He was a highly enthusiastic and long-time lecturer in many courses across departments, including BCH 401 (now called BCH 408), winning several teaching awards, and was course director of BCH 401 for many years. He also served as Associate Chair of the Biochemistry Department, and for almost 10 years served as Associate Dean and Director of the school-wide Graduate Program in Biology and Medicine, a forerunner to our current interdepartmental graduate programs. George was a great colleague, always pleasant and affable in his interactions with others. He will be greatly missed.
A serendipitous discovery and the choreographed dance of fragile X research
Tuesday, October 18, 2022
The choreography of development is a delicate dance. Beginning in utero, chromosomes, DNA, genes and RNA twirl, tap, and sashay their way in a precise pattern. A misstep or a missing step that changes the routine causes body and brain functions to go awry – as is the case with many intellectual and developmental disabilities (IDD). Fragile X syndrome is the most common known single-gene cause of inherited IDDs, including autism. Scientists know the misstep in this syndrome is in the gene FMR1. FMR1 is responsible for making the protein FMRP, which is necessary for typical brain development.
Lynne Maquat, Ph.D., founding director of the Center for RNA Biology at the University of Rochester, and professor of Biochemistry & Biophysics, Oncology, and Pediatrics, did not set out to study fragile X. It was through another line of research – her seminal discovery of and decades’ worth of work on nonsense-mediated mRNA decay (NMD) – that fragile X syndrome entered her radar. NMD is a cellular quality-control mechanism that plays a role in both healthy and disease states, and her lab discovered that it is overactive in people with fragile X.
“It was complete serendipity,” Maquat said. “No one ever thought to look at NMD and fragile X. So now we’re trying to figure out what happens at the molecular level when FMRP is absent; we want to understand the network of altered gene expression by identifying mis-regulated messenger RNAs (mRNAs).”
One of the most prominent surveillance systems in the body that protects against mistakes in gene expression that lead to disease, NMD is a complex pathway that is at the heart of many of the collaborations between Maquat and other University of Rochester scientists. Together, with funding from the National Institutes of Health (NIH) and the FRAXA Research Foundation, they aim to gain a deeper understanding of the sophisticated mechanisms related to NMD that will contribute to developing new drug therapies for genetic disorders such as fragile X syndrome, cystic fibrosis, and hundreds of others.
INTO THE BRAIN
Associate professor of Biomedical Genetics Christoph Pröschel, Ph.D., has spent much of his career interested in neurogenetic diseases that primarily affect the white matter of the brain, which carries signals throughout the organ. His lab started working with induced pluripotent stem cells (iPSCs) to understand different neural cell types, providing a solid foundation for their IDD research. “My lab and the Maquat lab have a mutual interest in the molecular mechanism of fragile X,” said Proschel. “It is key to finding any kind of hope for a future therapy.”
The Pröschel lab makes and differentiates neural stem cells that mimic fragile X syndrome, allowing his team to test hypotheses and understand how different therapies impact cell biology and function. He and Tatsuaki Kurosaki, Ph.D., research assistant professor in the Maquat lab, used these neural stem cells to understand the relationship between FMRP and NMD. They discovered that NMD controls the amounts of messenger RNAs deriving from a wide range of genes throughout the brain, including genes that govern motor control and cognitive processes related to attention, learning, and language. They also found that when FMRP is absent from cells, as it is in people with fragile X syndrome, NMD shifts into overdrive.
This work was part of a 2021 study published in Nature Cell Biology led by Maquat that revealed that tamping down NMD with small molecule inhibitors restored a large proportion of neurological functions in these cells.
Most recently, Pröschel co-authored research published in Molecular Cell led by Maquat and co-authored by Hana Cho, Ph.D., and Elizabeth Abshire, Ph.D., of her lab. The study highlighted a complex molecular dance between NMD and the enzyme AKT, which plays a key role in cell growth and survival. Both AKT and NMD are overactive in fragile X. Using neural stem cells that lack the FMRP protein, they tested a drug called Afuresertib, which inhibits AKT. They discovered that blocking AKT in the fragile X cells decreased its activity and decreased NMD. These cells then acted more like typical, non-disease cells.
There is still a lot the team doesn’t know about how AKT and NMD interact, because they both influence and regulate multiple activities in cells, but this work provides good direction that could inform the development of future treatments for fragile X syndrome.
“This has been one of the real fun chapters of my career – working with this group,” said Pröschel. “Everyone brings such a different perspective to the project.”
FROM SURGERY TO THE LAB
As a neurotologist (subspecialist of Otolaryngology), Hitomi Sakano, M.D., Ph.D., spends time in the clinic with patients with hearing issues or hearing loss. In the lab, she aims to understand how the brain adapts to sound information.
Her work with fragile X syndrome began as a resident at the University of Washington when she took interest in FMRP, which is highly expressed in the auditory brainstem nuclei of a typical brain and is the same protein missing in fragile X patients. When Sakano came to the Medical Center in 2018, she brought the fragile X mouse model to study this and joined the Center for RNA Biology.
“I also use the [knockout] mouse model to study hyperacusis – extreme sensitivity to sound,” said Sakano. “We know that fragile X patients have sensory and auditory sensitivity, so this model is a great tool to study both.” Hyperacusis is also very common in the general population (some report up to 15 percent) so understanding the mechanism could potentially impact our broader community.
Sakano hypothesizes that FMRP regulates genes that enable neuroplasticity to maintain
From left: Hitomi Sakano, M.D., Ph.D., and Lynne Maquat, Ph.D.
normal processing of auditory information. If true, there may be therapeutic targets for symptoms like auditory hypersensitivity in fragile X. Funding from the Schmitt Program in Integrative Neuroscience (SPIN) through the Del Monte Institute for Neuroscience Pilot Program and a NIH Research Career Development Award for clinician-scientists are supporting her research, which involves investigating the gene expression abnormalities in the auditory brainstem of the fragile X mouse model that might explain the auditory hypersensitivity in these mice. To date, she has found some interesting RNAs that encode synaptic proteins. These findings open up the possibility of targeting these genes for the treatment of hyperacusis.
She co-authored a study with the Maquat and Pröschel labs in Genome Biology. The research used the mouse model whose FMR1 gene is knocked-out. These findings build upon Maquat’s previous research that showed NMD hyperactivation in neuronally induced stem cells from fragile X patients. This hyperactivity negatively impacts many neuronal mRNAs important to brain development. The Genome Biology paper showed NMD goes into overdrive in the brain during early development in a mouse with fragile X. These researchers are now testing various therapeutics to inhibit NMD.
“Being able to collaborate to gain meaningful results to move this science forward is the value of being at an academic medical center like Rochester,” said Sakano. “These steps are what will ultimately lead to treatments and therapies that I use in the clinic someday to help my patients.”
ON THE HORIZON
Forthcoming research aims to broaden the scope of the fragile X work at the Medical Center. One of the world’s largest clinics for fragile X is in Israel, where an estimated 80 percent of women are screened for the inherited disease. Michael Telias, Ph.D., assistant professor of Ophthalmology, Neuroscience, and Center for Visual Science, began studying fragile X as a graduate student in Israel. He uses human embryonic stem cells that carry the mutation for fragile X to look inside neurons at the molecular and cellular levels to shed light on the human-specific mechanisms affected by this syndrome.
“Human neurons have shown us that these cells have a problem receiving information and communicating information to the next cell,” said Telias. “We cannot do this work in humans, so using human cells enables us to know what to target in the cell. That is the only way we will be able to develop treatments that work.”
In the Frederick J. and Marion A. Schindler Cognitive Neurophysiology Laboratory, research assistant professor Tufikameni Brima, Ph.D., is aiming to use electroencephalography (EEG) and event-related potentials (ERP) to better understand how the brains of patients with fragile X respond to various stimuli. This work has the potential to build upon the ongoing molecular research being conducted by Telias and others.
“Ultimately, what we are figuring out is what happens when FMRP is absent. We don’t know the whole story,” Maquat said. “However, FMRP is an RNA-binding protein, and in work soon to be published in Molecular Cell, Kurosaki and I have now defined those messenger RNAs that are normally bound by FMRP and how the absence of FMRP binding results in those mRNAs making too much protein. These results have allowed us to identify which genes are affected and how. Our work will pave the way for better therapeutics for those living with fragile X.”
Read More: A serendipitous discovery and the choreographed dance of fragile X researchBlanton Tolbert, PhD appointed as HHMI vice president and head of the Institute’s new Center for Science Leadership and Culture
Thursday, October 6, 2022
 Former Biophysics PhD student Blanton Tolbert, Ph.D. was recently appointed as the Howard Hughes Medial Institute’s Vice President and Head of the HHMI’s Center for Science Leadership and Culture. Dr. Tolbert is currently the Rudolph and Susan Rense Professor in the Chemistry Department at Case Western University, and Vice Dean of Diversity, Equity and Inclusion at CWRU School of Medicine. Dr. Tolbert’s research employs biophysical and structural methods to understand how viral RNAs and interact with critical proteins, with a emphasis on human immunodeficiency virus RNAs. More information regarding his appointment can be found here:
Former Biophysics PhD student Blanton Tolbert, Ph.D. was recently appointed as the Howard Hughes Medial Institute’s Vice President and Head of the HHMI’s Center for Science Leadership and Culture. Dr. Tolbert is currently the Rudolph and Susan Rense Professor in the Chemistry Department at Case Western University, and Vice Dean of Diversity, Equity and Inclusion at CWRU School of Medicine. Dr. Tolbert’s research employs biophysical and structural methods to understand how viral RNAs and interact with critical proteins, with a emphasis on human immunodeficiency virus RNAs. More information regarding his appointment can be found here:
https://www.hhmi.org/news/blanton-tolbert-named-hhmis-inaugural-vice-president-science-leadership-and-culture
Professor Lea Vacca Michel pens article in ASBMB Today on unexpected challenges in removing barriers to inclusion
Wednesday, August 24, 2022
 Lea Vacca Michel, PhD, an Associate Professor at the Rochester Institute of Technology, has written an insightful article on an unexpected challenge she experienced in conducting an excursion for her group to Universal Studios in Orlando Florida. Her research group was attending a scientific meeting in Orlando and wished to explore the theme park as a fun side trip. Michel’s article, in ASBMB Today, the member magazine of the American Society for Biochemistry and Molecular Biology, is a self-critical appraisal of the excursion describing her efforts to make sure activities were enjoyable for everyone, including two deaf/hard-of-hearing members of the group. Despite apparently successful similar excursions in the past and despite Michel’s best efforts at inclusivity, the unanticipated effects of the rides at the park made her aware of new challenges. The full article can be found here (https://www.asbmb.org/asbmb-today/careers/081122/all-alone-in-a-crowd?utm_source=Emailer). Lea is active in the ASBMB and is a member of the ASBMB Minority Affairs Committee (links to other articles by and about Lea, below). Lea received her Ph.D. from the Biophysics Program in the Department of Biochemistry and Biophysics in 2007 and has taught at RIT since 2009.
Lea Vacca Michel, PhD, an Associate Professor at the Rochester Institute of Technology, has written an insightful article on an unexpected challenge she experienced in conducting an excursion for her group to Universal Studios in Orlando Florida. Her research group was attending a scientific meeting in Orlando and wished to explore the theme park as a fun side trip. Michel’s article, in ASBMB Today, the member magazine of the American Society for Biochemistry and Molecular Biology, is a self-critical appraisal of the excursion describing her efforts to make sure activities were enjoyable for everyone, including two deaf/hard-of-hearing members of the group. Despite apparently successful similar excursions in the past and despite Michel’s best efforts at inclusivity, the unanticipated effects of the rides at the park made her aware of new challenges. The full article can be found here (https://www.asbmb.org/asbmb-today/careers/081122/all-alone-in-a-crowd?utm_source=Emailer). Lea is active in the ASBMB and is a member of the ASBMB Minority Affairs Committee (links to other articles by and about Lea, below). Lea received her Ph.D. from the Biophysics Program in the Department of Biochemistry and Biophysics in 2007 and has taught at RIT since 2009.
https://www.asbmb.org/asbmb-today/opinions/031022/if-i-don-t-who-will
https://www.asbmb.org/asbmb-today/people/120121/michel-strives-to-be-a-better-mentor
Lynne Maquat Receives Advisory Appointment at International Centre for Genetic Engineering and Biotechnology
Friday, August 12, 2022

 Lynne E. Maquat, Ph.D., the J. Lowell Orbison Endowed Chair and Professor of Biochemistry and Biophysics, Oncology and Pediatrics at the University of Rochester School of Medicine & Dentistry has been elected a member of the Council of Scientific Advisers (CSA) of the International Centre for Genetic Engineering and Biotechnology (ICGEB). Also the founding director of the University of Rochester’s Center for RNA Biology, Maquat will serve as a member of the Council for a term of three years, beginning in July 2022.
Lynne E. Maquat, Ph.D., the J. Lowell Orbison Endowed Chair and Professor of Biochemistry and Biophysics, Oncology and Pediatrics at the University of Rochester School of Medicine & Dentistry has been elected a member of the Council of Scientific Advisers (CSA) of the International Centre for Genetic Engineering and Biotechnology (ICGEB). Also the founding director of the University of Rochester’s Center for RNA Biology, Maquat will serve as a member of the Council for a term of three years, beginning in July 2022.
The ICGEB is an intergovernmental organization that runs over 45 state-of-the-art laboratories in Trieste, Italy, New Delhi, India and Cape Town, South Africa. If forms an interactive network with close to 70 member states, and plays a key role in biotechnology by promoting research excellence, training, and technology transfer to industry. The Council of Scientific Advisers is composed of fifteen “eminent” scientists who are active in the life sciences at the international level. Maquat will work together with fellow advisors to provide ICGEB member states with effective training programs and dedicated research projects.
With this appointment, Maquat has held a dozen international advisory positions since 2000. In addition to the ICGEB, she is currently a member of the Scientific Advisory Board for the Max Planck Institute for Molecular Genetics in Berlin, Germany and a member of the Medical Advisory Board for the Canada Gairdner International Awards and The Gairdner Foundation in Canada.
Maquat is the second member of the University of Rochester community to be elected a member of the Council of Scientific Advisers. Arthur Kornberg, M.D., who earned his medical degree from the School of Medicine & Dentistry in 1941 and went on to receive the Nobel Prize in Physiology or Medicine in 1959, served as a scientific advisor to the ICGEB from 1995 to 2005.
Double Duty: Early Research Reveals how a Single Drug Delivers Twice the Impact in Fragile X
Monday, June 27, 2022
Like many neurological diseases, there’s a lot we don’t understand about fragile X syndrome. But, after studying the disorder for several years, Lynne Maquat’s lab knew two important things: the enzyme AKT, which plays a key role in cell growth and survival, and the quality control pathway known as NMD (nonsense-mediated mRNA decay), are both in overdrive in fragile X.
In a new study in the journal Molecular Cell, the team reveals how these two major players interact, highlighting a complex molecular dance that could inform the development of future treatments for fragile X syndrome.
Two paths to pursue
AKT is a hub for cell signaling, helping cells communicate about important processes like cell growth, proliferation and protein production. When cells are stressed – for example, in cancer, diabetes, heart disease and neurological disorders, including fragile X – AKT can send too many (or too few) signals or messages as part of a cell survival mechanism.
NMD is like a molecular guide that helps our cells make smart decisions that (in most cases) improve cellular function and contribute to good health. For example, NMD supports gene expression by flagging and destroying mRNAs (messenger RNAs) that are carrying faulty genetic instructions that could lead to disease. It also helps our cells adjust to changes in development and in their environment, and more rapidly respond to certain stimuli.
Read More: Double Duty: Early Research Reveals how a Single Drug Delivers Twice the Impact in Fragile XThree Biochemistry and Biophysics Department Students Win Hooker Fellowships
Monday, June 13, 2022
 Congratulations to Justin Galardi, Matt Raymonda, and Griffin Schroeder, who won Elon Hooker Dissertation Fellowships for 2022-2023.
Congratulations to Justin Galardi, Matt Raymonda, and Griffin Schroeder, who won Elon Hooker Dissertation Fellowships for 2022-2023.
This fellowship was first endowed by the Hooker family in 1947 in memory of Mr. Elon Huntington Hooker, founder of the Hooker Chemical Company and a graduate of the University of Rochester. The fellowship supports graduate students across disciplines in the sciences, and thus is one of the University’s most competitive awards. The fellowship is given to students who display exceptional ability and promise in their dissertation research. Justin works in the Kielkopf lab, and studies the structure and function of early-stage splicing factors, Matt works in the Munger lab and studies metabolic vulnerabilities associated with viral infection, and Griffin is in the Wedekind lab and studies structural bases of translation regulation by riboswitches. These students carry on a long tradition of the Department of Biochemistry and Biophysics being well-represented among the annual Hooker awardees.
Congratulations to Justin, Matt and Griffin!
Dmitri Ermolenko awarded NIH MIRA Award
Wednesday, February 23, 2022
Congratulations to Dmitri Ermolenko on receiving prestigious NIH R35 MIRA (Maximizing Investigators' Research Award) grant. The goal of MIRA is to increase the efficiency of NIGMS funding by providing highly talented and promising investigators with greater stability and flexibility, thereby enhancing scientific productivity and the chances for important breakthroughs. The Ermolenko lab will use MIRA funding to investigate molecular mechanisms of translation by studying structural dynamics of the ribosome, and the role of mRNA secondary structure in translation regulation.
Ermolenko and Mathews groups publish in Nature Communications
Wednesday, February 23, 2022
A new study by the Ermolenko and Mathews groups, which is out in Nature Communications https://nature.com/articles/s41467-022-28600-5, shows that contrary to prevailing dogma, specific length and structure, rather than high stability, enable regulatory mRNA stem-loops to pause translation. This work was led by a talented BMB PhD student Chen Bao.
Congratulations to Chen and colleagues!
Convince us why your favorite RNA or RNA-binding protein is worthy of our admiration
Friday, January 28, 2022
Department of Biochemistry & Biophysics Seminar Series Participants,
Thank you to those who participated in and/or viewed the UR Center for RNA Biology’s RNA Presentations on Jan 12th and 26th, sponsored by the RNA Society, Lexogen, and the UR Center for RNA Biology. The judging committee was impressed with the quality of the abstracts submitted and the selected presentations given by UR graduate students and research staff with the prompt: “Convince us why your favorite RNA or RNA-binding protein is worthy of our admiration”.
Each of the oral presenters, who were chosen based on their quality of their abstracts, will be receiving a one-year membership to the RNA Society. Three presenters will also receive prize funds of $300 each.
The three presenters to be awarded a one-year membership to the RNA Society and $300 ea., in alphabetical order, are:
Xueyang He (presented Jan 12th ) - Biophysics Grad Student, Boutz Lab, Biochemistry & Biophysics
“Modeling the effects of cancer-associated spliceosome mutations and identifying driving intronic features using deep-learning neural networks”
Adrián Moisés Molina Vargas (presented Jan 26th) - Genetics Graduate Student, O'Connell Lab (Biochemistry & Biophysics), Biomedical Genetics
“From prokaryote immunity to the newest RNA targeting tool. Unveiling the nature and opportunities of the Cas13 CRISPR RNA-nuclease”
Li Xie (presented Jan 26th) - Genetics Graduate Student, Pröschel Lab, Biomedical Genetics
“Deciphering eIF2B deficiencies in a neurodegenerative disorder”
Honorable mention presenter to receive a one-year membership to the RNA Society
Perinthottathil Sreejith, PhD (presented Jan 12th ) - Staff Scientist, Bharadwaj Lab, Pathology & Laboratory Medicine, who presented on his previous work as a Postdoc in the Biteau Lab in Biomedical Genetics
“Imp interacts with Lin28 to regulate adult stem cell proliferation in the Drosophila intestine”
Again, thank you all for contributing to make our contest interesting and exciting.
Liz - On behalf of Lynne Maquat, PhD (Director, UR Center for RNA Biology)
Read More: Convince us why your favorite RNA or RNA-binding protein is worthy of our admirationDepartment of Biochemistry and Biophysics Annual Toy Drive
Wednesday, January 19, 2022
 The Department of Biochemistry and Biophysics held its annual toy drive this season to collect toys and items for the Golisano Children's Hospital. Dr. Alan Grossfield delivered a cart full of goodies to the Golisano Children's hospital on Thursday, December 16th along with graduate student, Emily Robinson, and staff accountant, Amy Roman. The gifts are given to the children in the hospital during the holiday season. Any remaining gifts are used to support the needs of the children and playrooms throughout the year.
The Department of Biochemistry and Biophysics held its annual toy drive this season to collect toys and items for the Golisano Children's Hospital. Dr. Alan Grossfield delivered a cart full of goodies to the Golisano Children's hospital on Thursday, December 16th along with graduate student, Emily Robinson, and staff accountant, Amy Roman. The gifts are given to the children in the hospital during the holiday season. Any remaining gifts are used to support the needs of the children and playrooms throughout the year.
Read the thank you letter.
In the Pocket: RNA Binding Discovery Supports ‘RNA World’ Theory of Early Life on Earth
Friday, January 14, 2022
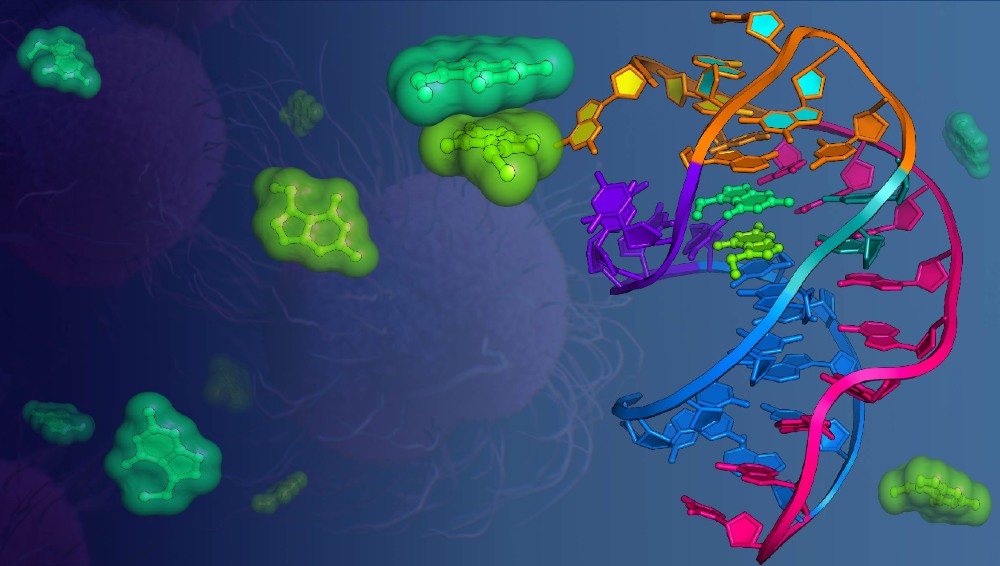

RNA biologists at the University of Rochester Medical Center (URMC) have discovered that RNA, the chemical cousin of DNA, can bind two metabolites (small molecules) at the same time in a single binding pocket, causing those molecules to interact. This discovery, published in Nature Communications this week, could lead to new antibacterial drugs while helping to fill a gap in the controversial “RNA world” theory, which suggests that RNA molecules enabled life to evolve on Earth 3.5 billion years ago.
Read More: In the Pocket: RNA Binding Discovery Supports ‘RNA World’ Theory of Early Life on Earth