Welcome to the Kim Lab

Optical imaging of immunity
Host immune response against infection and inflammation:
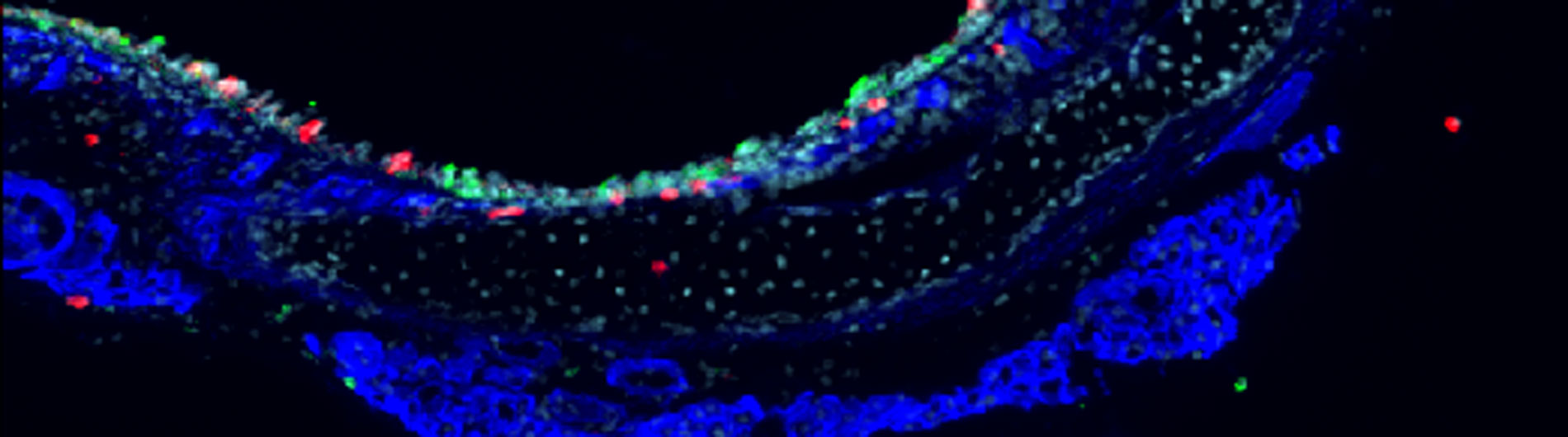
A) The maintenance of homeostatic immune surveillance and the development of effective adaptive immune responses require that T cells cross tissue barriers and move throughout the body, migrating in and out of the bone marrow, lymphoid and non-lymphoid tissues, under both normal and infected or inflamed conditions. The efficient trafficking of activated effector T cells into peripheral non-lymphoid tissues is key to enact their protective functions. Insufficient T cell activity contributes to impaired local adaptive immunity, recurrent infections and delayed wound healing, whereas excessive T cell recruitment leads to an exaggerated inflammatory response and the associated tissue damage. Importantly, successful early local innate immune response is critical for elicitation of T cell effector functions at the peripheral tissue sites. Therefore, it is likely that the type of innate cells, mode of early innate responses, and associated local inflammatory mediators will all impact on the molecular mechanisms by which effector T cells successfully move into the inflamed tissues. Our objective is to determine how the early innate immune responses prime and remodel the tissue microenvironment prior to effector T cell homing and how the tissue-specific inflammatory cues further modify the chemokine signals, integrin activation and migration of T cells.
B) Severe sepsis, a systemic inflammatory response to infections associated with acute organ dysfunction, is an increasing cause of morbidity and mortality among children and adults. Neutrophil recruitment during sepsis is key for host defense against infection and injury, and precise regulation of the interaction between neutrophils and components of the vascular layer is essential to minimize excessive damage to vascular walls during a localized inflammatory response, thus to sustain vascular homeostasis and control microvessel permeability. During severe sepsis, however, this system fails and massive infiltration of active neutrophils followed by exaggerated inflammatory response participates in the pathogenesis of multi-organ system failure (MOSF) through vascular injury and host tissue damage. We will identify the molecular events that cause destruction of each vascular barrier during neutrophil recruitment and develop novel therapeutic approaches to restore homeostasis.
Optical control of immunity
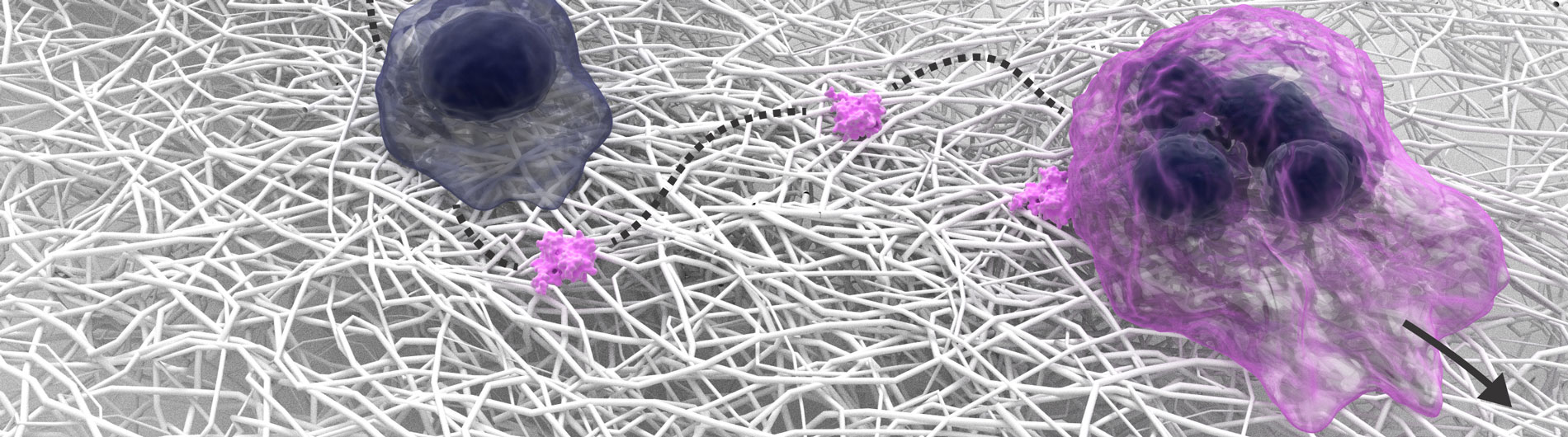
Cancer immunotherapy: Immunotherapy for cancer is an exciting topic, as it involves stimulating a patient’s own immune system to fight the malignancy. We are using a process called “optogenetics” to try to improve the response rates for immunotherapy. Three underlying issues challenge immunotherapy: First, cancer cells are smart and evasive, often hiding from the immune system. Second, tumors suppress the immune system, presenting another challenge for the anti-cancer T cells once they’ve located a tumor. Third, immunotherapy often comes with side effects. We are studying light and optics as a tool to bypass all three obstacles. All cytolytic T cells (e.g. CD8 T cells) contain effector molecules that can directly kill cancer cell. To make sure the T cells know where to find their target, Our lab has engineered T cells that respond to light. By implanting a light source — an LED chip that our group also invented — into the body of a mouse, we demonstrated that light can guide the T cells to the cancer cells. Once there, the light source also boosts the toxic, biological reaction within the T cells, better positioning them to wipe out their target cancer. And finally, we will invent a molecular safety switch (also based on light) that causes T cells to self-destruct when their job is done, preventing severe side effects resulting from the immune system remaining in overdrive. The achievement of functional hybrid photosensitive receptors represents a real tour de force, and opens up many analytical and therapeutic possibilities. This is particularly important because of the increasing success of cancer immunotherapies – Our optogenetics approaches will provide further capabilities to such approaches.

Minsoo Kim, Ph.D.
Principal Investigator
Projects
View All ProjectsSelected Publications
M. Kim, C.V. Carman, and T.A. Springer. 2003. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301(5640), 1720-1725.
N.A. Morin, P.W. Oakes, Y.-M. Hyun, D. Lee, E.Y. Chin, M.R. King, T.A. Springer, M. Shimaoka, J.X. Tang, J.S. Reichner, and M. Kim. 2008. Non-muscle myosin heavy chain-IIA (My-H9) mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J. Exp. Med. 205(1):195-205.
G.F. Elphick, P.P. Sarangi, Y-M Hyun, J.A. Hollenbaugh, A. Ayala, W.L. Biffl, H-L Chung, A.R. Rezaie, J.L. McGrath, D.J. Topham, J.S. Reichner, and M. Kim. 2009. Recombinant human activated protein C inhibits integrin-mediated neutrophil migration. Blood 113(17): 4078-85 .
CM Baker, WA Comrie, Y-M Hyun, C Fedorchuk, C Brakebusch, J Miller, M Meier-Schellersheim, and Kim M. 2012. Opposing roles for RhoH GTPase during T cell migration and activation. Proc Natl Acad Sci U S A Jun 26;109(26):10474-9.
Y-M Hyun, R Sumagin, P Sarangi, M Overstreet, C Baker, D Fowell, I Sarelius, and M. Kim. 2012. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J. Exp. Med. Jul 2;209(7):1349-62.
Sarangi PP, Lerman YV, Lim K, Hyun Y-M, Falkner KL, Pietropaoli AP, Lacy-Hulbert A, Sonnenberg A, and Kim M. 2014. Sepsis lethality through exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is dependent on integrin α3β1. Blood Dec 4;124(24):3515-23.
Xu Y, Hyun YM, Lim K, Lee H, Cummings RJ, Gerber SA, Bae S, Cho TY, Lord EM, and Kim M. 2014. Optogenetic control of chemokine receptor signal and T-cell migration. Proc Natl Acad Sci U S A Apr 29;111(17):6371-6.
Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, Topham DJ, and Kim M. 2015. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science Sep 4; 349 (6252): aaa4352.
Kim KD, Bae S, Capece T, Nedelkovska H, de Rubio RG, Smrcka AV, Jun CD, Jung W, Park B, Kim TI, Kim M. (2017 May 15). Targeted calcium influx boosts cytotoxic T lymphocyte function in the tumour microenvironment. Nature communications.
Lim K, Kim T-H, Trzeciak A, Amitrano AM, Reilly EC, Prizant H, Fowell DJ, Topham DJ, and & Kim M. (2020 Aug 3). In situ neutrophil efferocytosis shapes T cell immunity to influenza infection. Nature Immunol.
Fowell DJ, Kim M. (2021 Feb 24). The spatio-temporal control of effector T cell migration.. Nature reviews Immunology.
Hong Y, Walling BL, Kim HR, Serratelli WS, Lozada JR, Sailer CJ, Amitrano AM, Lim, Mongre RK, Kim KD, Capece T, Lomakina EB, Reilly NS, Vo K, Gerber SA, Fan TC, Yu LT, Oakes PW, Waugh RW, Jun CD, Reagan PM, & Kim M. (2023 April 17). St3gal1 and βII-spectrin pathways control CAR-T cell migration to target tumors. Nature Immunology
View All PublicationsNews
Affiliations
- Microbiology & Immunology
- Otolaryngology
- Pharmacology & Physiology
- David H. Smith Center for Vaccine Biology and Immunology
- James P. Wilmot Cancer Center
- NIH T32 Training Grant in Cellular, Biochemical & Molecular Sciences
- NIH T32 Training Grant in Immunology
- Pulmonary T32 Training Grant
- Cellular and Molecular Pharmacology and Physiology Ph.D. Program
- Immunology, Microbiology, and Virology Ph.D. Program
- Master’s Degree in Pharmacology
- Master’s Degree in Physiology
- PhD Program in Pathology - Cell Biology of Disease
- Translational Biomedical Science Ph.D. Program
- Center for Tumor Immunology Research
- Program for Advanced Immune Bioimaging
September 11, 2024
Research: How the Immune System Fails as Cancer Arises
April 17, 2023
Wilmot Researchers Discover How to Steer Army of Immune Cells toward Cancer
July 5, 2022
Researchers Receive $2.5 million NIH Grant to Study Potential Sepsis Drug Therapies
November 20, 2020
Congratulations Dr. Kim
Contact Us
Minsoo Kim Lab
KMRB 3-9858
601 Elmwood Ave,
Rochester, NY 14642