About the New LINX™ Reflux Management System
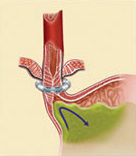
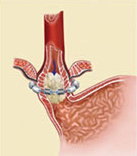
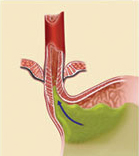
The LINX Reflux Management System is composed of a series of titanium beads, each with a magnetic core, connected together with independent titanium wires to form a ring shape. The LINX device is implanted at the lower esophageal sphincter (LES), a circular band of muscle that closes the last few centimeters of the esophagus and prevents the backward flow of stomach contents.
The force of the magnetic beads is designed to provide additional strength to keep a weak LES closed. Upon swallowing, the magnetic force between the beads is overcome by the higher pressures of swallowing forces – and the device expands to accommodate a normal swallow of food or liquid. Once the food passes though the LES, the device returns to its resting state.
The LINX device, which is about the size of a nickel, can be inserted laparoscopically (through tiny incisions) in less than an hour. As one of 15 specialized clinical trial sites across the U.S. and Europe, URMC has been implanting the device in study patients since 2009.
It is important to note that patients with a LINX device will no longer be able to undergo Magnetic Resonance Imaging (MRI) procedures. The magnetic beads interfere with the machine and can cause the device to be damaged and the patient to be injured.
Meet Our Surgeons
Christian Peyre, MD, Associate Professor at URMC
FAQs – LINX™ at a Glance

What Is It?
The LINX Reflux Management System (LINX device) consists of a series of titanium beads, each with a magnetic core, connected together with titanium wires to form a ring shape. The LINX device is surgically implanted around the lower end of the esophagus. It is used to treat gastroesophageal reflux disease (GERD) in patients who continue to have GERD symptoms despite the use of maximum medical therapy for the treatment of their reflux.
How Does It Work?
The LINX device is implanted around the lower esophageal sphincter to strengthen a weak sphincter. Using the device helps to prevent the contents of the stomach from backing up into the esophagus (reflux). By restricting the flow of stomach contents into the esophagus, a patient’s heartburn and/or reflux/regurgitation systems will improve and he or she may no longer require medicines to treat these symptoms.
Even though the LINX device helps to prevent stomach contents from flowing back into the esophagus, it does not prevent movement of food or liquids down the esophagus into the stomach. When the patient swallows, the pressure in the esophagus increases and the magnetic beads move apart on the titanium wires. As the beads move apart, the magnetic force decreases. This separation of the beads allows food or liquids to pass normally into the stomach. After the food or liquids have passed into the stomach, the magnetic beads return to the closed position.
When Is It Used?
The LINX device is used in patients who continue to have symptoms of GERD, such as heartburn and reflux, despite maximum medical therapy (daily use of medicines such as proton pump inhibitors). It is intended to be used by patients who would be considered candidates for anti-reflux surgery.
What Will It Accomplish?
The LINX device will help prevent abnormal amounts of acid from the stomach moving back into the esophagus. In a clinical study of 100 patients implanted with the LINX device, 64 patients either had normal amounts of acid in the esophagus or at least a 50% improvement in the amount of acid in the esophagus. Additionally, 92 patients had improvement in their GERD symptoms, so they felt better. The clinical study also found that 93 patients were able to reduce their medicines or no longer needed to take any medicines to treat their GERD symptoms.
Are There Any Adverse Effects?
The most common adverse effect experienced by patients was difficulty swallowing and some of the patients had their esophagus expanded (dilated). The second most common adverse effect was discomfort.
When Should It Not Be Used?
The LINX device should not be used in anyone who may be allergic or is allergic to titanium, stainless steel, nickel or iron (ferrous) materials.
Patients who have the LINX device should not be exposed to, or undergo, Magnetic Resonance Imaging (MRI). Exposure to MRI could cause serious injury to the patient and the device may be damaged.
Contact Us
For more information or to determine if you are a candidate for the LINX device, please call 585-275-1509. Patients may also ask for a referral from their primary care provider or GI specialist.
Please Note:
Patients will be asked to complete an initial screening, followed by pH, motility and other GI function testing, before being declared eligible candidates.
Not all insurance carriers cover procedures utilizing the LINX Reflux Management System. Patients filing through insurance should contact their carrier prior to their visit to verify coverage.