Research Laboratories
Investigators within the Department of Pathology and Laboratory Medicine are active in basic, translational and clinical research. Our faculty participate in interdepartmental and multidisciplinary research projects that explore topics including cellular and molecular pathology of bladder, prostate and breast cancer, blood group antigens, bone diseases, diabetes, hematopoiesis, immune disorders, lipid metabolism, kidney diseases, lymphomas and leukemias, neurodegenerative diseases and cardiovascular disease.
About Our Research Facilities
Our facilities include equipment for confocal and electron microscopy, flow cytometry and cell analysis, immunohistochemistry, microarray construction, laser microdissection and morphometric and image analysis.
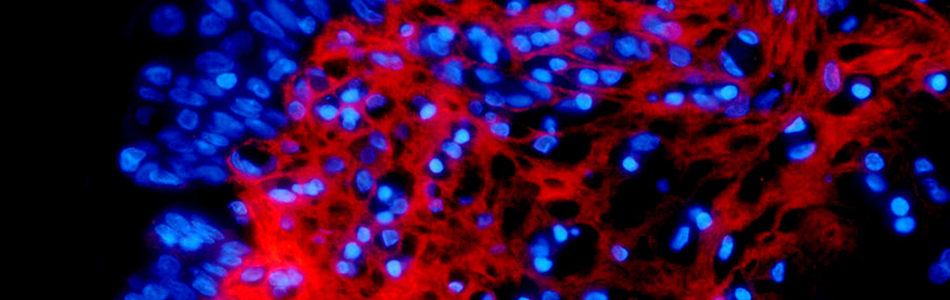
Improving diagnosis and treatment by investigating the cell autonomous and micro-environmental regulation of cancer, with particular focus on hematopoietic and urologic/reproductive tumors.
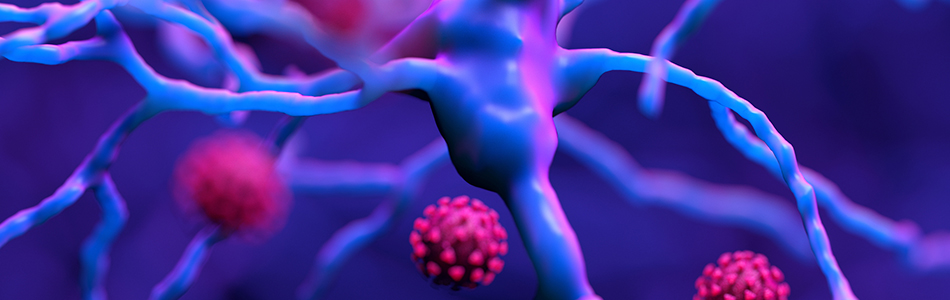
Exploring the pathology of inherited genetic disorders as well as cardiovascular, immunologic and neurodegenerative diseases.
Applying state of the art imaging techniques to a variety of disease states, including infectious and metabolic diseases.
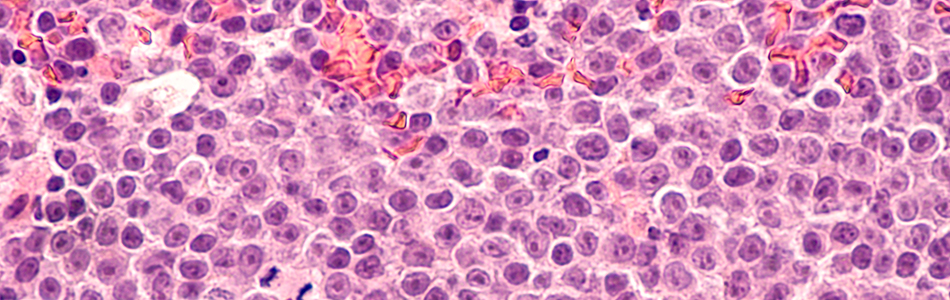
Studying normal function and molecular/cellular pathology in disorders of the blood and autoimmune diseases.
Examining regulation of bone and soft tissue cell function, loss, and regeneration in the context of aging and inflammatory conditions.
Collaborate with Us
We support collaborative efforts that create bridges between researchers and clinical specialists. Many are involved in clinical laboratory testing, clinical trials testing and basic scientific research. Our goals are to use new information from the research laboratory in order to enhance clinical testing; to use knowledge gained in the examination of clinical specimens in order to develop new hypotheses for basic research; and to share our knowledge through effective educational programs.