URMC / Labs / Benoit Lab / Projects / Developing Tissue Mimetics of the Outer Retinal Blood Barrier
Developing Tissue Mimetics of the Outer Retinal Blood Barrier
Project Collaborators:
Dr. Regine Choe, Dr. Jinjiang Pang, Dr. Ruchira Singh
The outer blood retinal barrier, which is composed of the retinal pigment epithelium (RPE) and its underlying vascular support, the choriocapillaris (CC), are the primary site of eye disease pathogenesis. This includes age-related macular degeneration (AMD), the leading cause of adult blindness in the US. Previous studies evaluating the role of RPE-CC complex in retinal diseases such as AMD have primarily relied on animal models. However, because the RPE-CC complex is a hierarchical functional unit in vivo, it has been difficult to evaluate the specific contribution of each individual tissue layer (RPE and CC) in disease development. Therefore, the goal of this project is to develop a functional in vitro tissue mimetic of the RPE-CC structure. Specifically, we will exploit human induced pluripotent stem cell (hiPSC) technology and tissue engineering to establish functional tissue mimetics. Patient-derived hiPSCs offer a unique platform to interrogate underlying pathology and disease-associated physiology of eye dysfunction. Furthermore, because of their modularity, hiPSC-derived in vitro models allow the flexibility to study the role individual cell type(s) and intercellular interaction in disease pathophysiology. We hypothesize that tissue morphogenesis of hiPSC-derived cells coordinated through hierarchical, biochemically-modified PEG hydrogels will recapitulate structure and function of in vivo RPE-CC complex. Our preliminary data suggests the tissue mimetic is feasible yet several improvements must be made to establish a functional mimetic. For example, the RPE layer in this co-culture system includes many discontinuities and exhibits poor longevity. To improve the mimetic, our aims are to 1) Develop tissue mimetics of the outer retinal blood barrier through (A) biochemical modification and (B) spatially organized culture of hiPSC-RPE and hiPSC-derived vascular networks enabled by PEG hydrogels and 2) Evaluate structure and function of RPE-CC tissue mimetics compared to in vivo tissue. Our expertise with engineering biomaterials to regulate the cell environment (Benoit), differentiating hiPSC to obtain the desired cell types (Singh), and angiogenesis (Pang) is crucial to establishing a complex cell model of RPE-CC complex using patient-derived cells. Successful completion of these aims would be a significant step towards ocular disease modeling and subsequent development of novel drug therapies for several retinal degenerative diseases, including age-related macular degeneration (AMD).
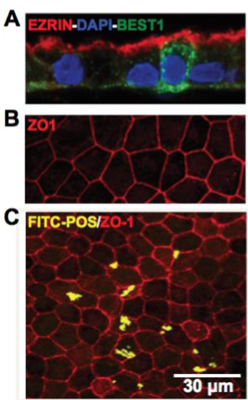
In the figure to the right, retinal barrier tissue mimetics exhibit morphological and functional similarities to native RPE. Representative confocal images showing A) polarized expression of RPE signature proteins, EZRIN and BEST1, with EZRIN localized on the apical surface and BEST1 found primarily on the basolateral surface, B) expression of tight junction protein, ZO1, and C) phagocytosis of FITC-labeled POS by hiPSC-RPE cells layers within tissue mimetics. hiPSC-RPE cells are counterstained with ZO1 to illustrate cell boundaries to ensure cellular uptake. Benoit and Singh unpublished data.
« back to all projects