News
20242023202220212020
The Department of Biochemistry and Biophysics announces that the NMR facility for structural biology has been decommissioned
Monday, December 18, 2023
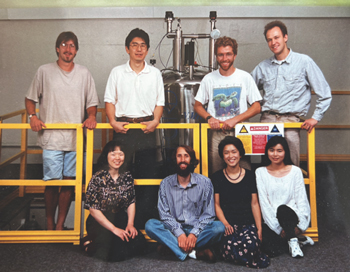
Shohei Koide (top, second from left) and his lab, along with NMR Center Director Scott Kennedy (Bottom, second from left), just after installation, 1996
The facility was originally installed in 1996 by Professor Shohei Koide with help from Scott Kennedy. The facility was key to Prof. Koide establishing a strong research program that included unique biophysical characterizations of proteins and valuable tools for targeted therapeutics. Over the years the experiments performed on the instrument have contributed to over 75 publications and ~22 structure entries in the Protein Data Bank with the involvement of dozens of primary investigators. Dr. Kennedy has maintained the instrumentation and collaborated with investigators and students since it was first opened, until the decommissioning. The facility contained a Varian Unity INOVA 600 MHz NMR spectrometer equipped with four RF channels, pulsed field gradients and triple resonance probes – state of the art features at the time. A 400 MHz NMR spectrometer (previously decommissioned) was also available in the facility as well as workstations and software for data analysis and structure calculation. The decision to discontinue the facility was influenced by an overall decline of interest in structural NMR, limited supplies and high cost of helium, a national trend toward centers, and retirement of Dr. Kennedy.
View more images from the NMR
O’Connell Comments on First FDA-Approved CRISPR-Based Therapy
Wednesday, December 13, 2023
 The FDA has just approved the use of CRISPR-based gene editing in the treatment of sickle-cell disease. Mitchell O’Connell, an assistant professor in the Department of Biochemistry and Biophysics, trained with Jennifer Doudna, who won the Nobel Prize for developing this technology. In a comment linked below, he discusses the approval and its implications for other treatments currently in development.
The FDA has just approved the use of CRISPR-based gene editing in the treatment of sickle-cell disease. Mitchell O’Connell, an assistant professor in the Department of Biochemistry and Biophysics, trained with Jennifer Doudna, who won the Nobel Prize for developing this technology. In a comment linked below, he discusses the approval and its implications for other treatments currently in development.
Read More: O’Connell Comments on First FDA-Approved CRISPR-Based TherapySayeeda Zain Fall travel award winners
Wednesday, December 13, 2023
We are pleased to announce the winners of the Sayeeda Zain Fall Travel awards: Gloria Asantewaa, Hannah Clary, Ashlin Poruthoor and Nick Rugelis.
The Sayeeda Zain Travel Award honors the distinguished career and charitable life of Dr. Sayeeda Zain. The award is given in the fall and spring semesters in recognition of research excellence to support travel and related expenses for attendance at a scientific conference or corporate internship to gain practical experience. The next round of Sayeeda Zain Travel Awards will be offered in Spring 2024.




Thank you to all those who applied and congratulations to Gloria, Hannah, Ashlin and Nick!
Elizabeth Abshire Awarded K99 Grant
Thursday, December 7, 2023
 Elizabeth Abshire, a postdoctoral researcher in the laboratory of Lynne Maquat, has been awarded a K99 grant by the National Institutes of Health. Her proposal, entitled “Regulation of exon junction complex composition and assembly”, is directed toward study of the formation of a key determinant of RNA decay.
Elizabeth Abshire, a postdoctoral researcher in the laboratory of Lynne Maquat, has been awarded a K99 grant by the National Institutes of Health. Her proposal, entitled “Regulation of exon junction complex composition and assembly”, is directed toward study of the formation of a key determinant of RNA decay.
Congratulations to Elizabeth on this significant achievement!
Joshua Munger Receives Outstanding Graduate Program Director Award
Monday, December 4, 2023
 Professor Joshua Munger of the Department of Biochemistry and Biophysics has been honored with the Outstanding Graduate Program Director Award by the Office of Graduate Education and Postdoctoral Affairs of the School of Medicine and Dentistry. This award recognizes individuals who have made exceptional or unique contributions to a graduate program and to the graduate student experience within that program. It includes an award of $2500, designated as unrestricted education support funds in aid of the graduate program.
Professor Joshua Munger of the Department of Biochemistry and Biophysics has been honored with the Outstanding Graduate Program Director Award by the Office of Graduate Education and Postdoctoral Affairs of the School of Medicine and Dentistry. This award recognizes individuals who have made exceptional or unique contributions to a graduate program and to the graduate student experience within that program. It includes an award of $2500, designated as unrestricted education support funds in aid of the graduate program.
The Award was presented on October 30th, at the Graduate Education and Postdoctoral Affairs Awards and Philosophy Meeting. Congratulations to Josh on this important and well-deserved award!
Hannah Clary Receives Outstanding Poster Presentation Award
Thursday, November 23, 2023
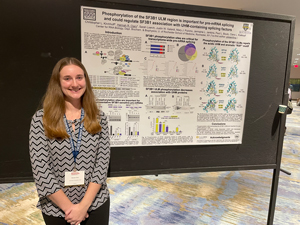 Hannah Clary, a student and T32 trainee in Clara Kielkopf’s group, was the recipient of a signal honor in November. She attended the inaugural FASEB conference on RNA Processing in Cancer: From Bench to Bedside. This meeting brings together cancer researchers, RNA scientists, clinicians and drug development companies to accelerate the transfer of fundamental advances into therapeutic use. The conference included invited talks, as well as a poster session. Hannah exhibited a poster entitled “Phosphorylation of the SF3B1 ULM region is important for pre-RNA splicing and could regulate SF3B1 association with UHM-containing splicing factors”. She was selected by a vote of the conferees to receive the Outstanding Presentation Award.
Hannah Clary, a student and T32 trainee in Clara Kielkopf’s group, was the recipient of a signal honor in November. She attended the inaugural FASEB conference on RNA Processing in Cancer: From Bench to Bedside. This meeting brings together cancer researchers, RNA scientists, clinicians and drug development companies to accelerate the transfer of fundamental advances into therapeutic use. The conference included invited talks, as well as a poster session. Hannah exhibited a poster entitled “Phosphorylation of the SF3B1 ULM region is important for pre-RNA splicing and could regulate SF3B1 association with UHM-containing splicing factors”. She was selected by a vote of the conferees to receive the Outstanding Presentation Award.
Congratulations to Hannah on this achievement!
Eric Phizicky Spotlighted by the RNA Society
Wednesday, November 8, 2023
 The RNA Society has posted a Spotlight article about Eric Phizicky, highlighting his research and his outstanding career at the University. In a comment on this well-deserved notice, Mitch OConnell tweeted [or possibly X'ed] the following: "Love this! Eric has been instrumental helping me stay afloat starting a lab at Rochester. Sitting down almost high school English teacher style going line by line, pen and paper through a grant application has been transformative about how I think to communicate. A true gift!"
The RNA Society has posted a Spotlight article about Eric Phizicky, highlighting his research and his outstanding career at the University. In a comment on this well-deserved notice, Mitch OConnell tweeted [or possibly X'ed] the following: "Love this! Eric has been instrumental helping me stay afloat starting a lab at Rochester. Sitting down almost high school English teacher style going line by line, pen and paper through a grant application has been transformative about how I think to communicate. A true gift!"
Congratulations to Eric on this recognition!
Read More: Eric Phizicky Spotlighted by the RNA SocietyLeah Brown Elected To Genesee Staff Council
Thursday, October 26, 2023
 Leah Brown, Assistant Graduate Studies Coordinator in the Department, has been elected to the Genesee Staff Council. The mission of the Council is to facilitate active and direct communication between University staff and senior administration, and to provide a forum for input and discussion of issues important to the staff and the University. The Council is composed of a group of diverse staff from across various University offices and academic departments.
Leah Brown, Assistant Graduate Studies Coordinator in the Department, has been elected to the Genesee Staff Council. The mission of the Council is to facilitate active and direct communication between University staff and senior administration, and to provide a forum for input and discussion of issues important to the staff and the University. The Council is composed of a group of diverse staff from across various University offices and academic departments.
Congratulations, Leah!
OyaGen Scientist Awarded SBIR Grant
Friday, October 13, 2023

 Jason D. Salter (Ph.D. URMC ’10), scientist working with Dr. Harold C. Smith at OyaGen, Inc (www.oyageninc.com) has been awarded as PI an NIH SBIR phase I two year grant for $600,000 to conduct high throughput, high content screening for a novel therapeutic that will prevent the Ebola virus from assembling infectious virions. The NIH award enabled the company to purchase a 100,000 compound diversity putlibrary and an Agilent Cytation high content, high through cell analyzer for drug discovery. A live cell assay for Ebola VP40 capsid assemble has been optimized with which compound library screening will be conducted this fall.
Jason D. Salter (Ph.D. URMC ’10), scientist working with Dr. Harold C. Smith at OyaGen, Inc (www.oyageninc.com) has been awarded as PI an NIH SBIR phase I two year grant for $600,000 to conduct high throughput, high content screening for a novel therapeutic that will prevent the Ebola virus from assembling infectious virions. The NIH award enabled the company to purchase a 100,000 compound diversity putlibrary and an Agilent Cytation high content, high through cell analyzer for drug discovery. A live cell assay for Ebola VP40 capsid assemble has been optimized with which compound library screening will be conducted this fall.
Biochemistry Professor’s Company Develops Potential Drug for Ebola and COVID
Thursday, October 12, 2023

 Professor Harold C. Smith, founder and CEO of OyaGen, Inc (www.oyageninc.com) and Ryan P. Bennett, Chief Scientific Officer at OyaGen (Biochemistry Ph.D. URMC ’07) have developed a therapeutic lead with highly potent antiviral activity for viruses that rely on RNA dependent RNA polymerases for their replication such as COVID, Ebola and Lassa virus. The preclinical data have been recently published in Antiviral Research (PMID: 37690700 DOI: 10.1016/j.antiviral.2023.105716) and two patents for the lead compound as a therapeutic for COVID and Ebola have been issued this year. OyaGen is conducting NIH grant funded antiviral efficacy testing in a hamster lung model as the last of four preIND studies recommended by the FDA prior to consideration of regulatory approval for Phase I clinical trials.
Professor Harold C. Smith, founder and CEO of OyaGen, Inc (www.oyageninc.com) and Ryan P. Bennett, Chief Scientific Officer at OyaGen (Biochemistry Ph.D. URMC ’07) have developed a therapeutic lead with highly potent antiviral activity for viruses that rely on RNA dependent RNA polymerases for their replication such as COVID, Ebola and Lassa virus. The preclinical data have been recently published in Antiviral Research (PMID: 37690700 DOI: 10.1016/j.antiviral.2023.105716) and two patents for the lead compound as a therapeutic for COVID and Ebola have been issued this year. OyaGen is conducting NIH grant funded antiviral efficacy testing in a hamster lung model as the last of four preIND studies recommended by the FDA prior to consideration of regulatory approval for Phase I clinical trials.
CannaMetrix Develops Potency Estimation System for Cannabinoids
Thursday, October 12, 2023

 DBB professor Harold C. Smith, founder and CEO of CannaMetrix, LLC (https://cannametrix.org/) and Charles O. Wolffsmith (Ph.D. ’18 URMC) have developed quantitative human cell-based potency for anticipating the biological potency of cannabinoids and blends of cannabinoids in the human body. Current cannabis industry standards only estimate potency using chemical composition analysis of cannabinoid products. CannaMetrix developed the EC50 Reporter assay in recognition that blends of cannabinoids have complex effects on the endocannabinoid system and that potency is a biological phenomenon. A patent for the CannaMetrix cell-based potency assay has been issued this year. Assay development has been supported by XXII Century Group out of Buffalo, NY through $2+ million licensing agreement. Current studies use the EC50 Reporter assay to assess the potency of commercial blends of synthetic cannabinoids and as a guide for developing new varieties of Cannabis.
DBB professor Harold C. Smith, founder and CEO of CannaMetrix, LLC (https://cannametrix.org/) and Charles O. Wolffsmith (Ph.D. ’18 URMC) have developed quantitative human cell-based potency for anticipating the biological potency of cannabinoids and blends of cannabinoids in the human body. Current cannabis industry standards only estimate potency using chemical composition analysis of cannabinoid products. CannaMetrix developed the EC50 Reporter assay in recognition that blends of cannabinoids have complex effects on the endocannabinoid system and that potency is a biological phenomenon. A patent for the CannaMetrix cell-based potency assay has been issued this year. Assay development has been supported by XXII Century Group out of Buffalo, NY through $2+ million licensing agreement. Current studies use the EC50 Reporter assay to assess the potency of commercial blends of synthetic cannabinoids and as a guide for developing new varieties of Cannabis.
Biochemistry Ph.D Student Madeline Jensen Receives Perfect Score on NIH Fellowship Application
Tuesday, September 5, 2023

Madeline Jensen in Israel in early August
Congratulations to third-year BMB Graduate Student, Madeline Jensen, in getting a fundable score on her NIH F31 submission entitled ‘Identification and structural basis of a potential licensing factor of the Integrator cleavage module’. Ms. Jensen received the top possible score on her application that can be assigned by an NIH review panel. This three-year NIH pre-doctoral fellowship will support her thesis project in the Wagner lab where she will continue her exciting studies of BRAT1, Integrator, and& potential connections to DNA damage. She also recently attended the ‘RNA Summer School’ held in Jerusalem, an NSF-BSF-funded two-week lab-intensive course.
Congratulations Maddie!
UR Center for RNA Biology Members and Alumni Attend RNA 2023 in Singapore
Friday, June 9, 2023

Pictured above, from left to right: Jessica Perciaccante (Genetics Grad Student, Anderson Lab); Douglas Anderson, PhD (Assistant Professor, Medicine, Aab CVRI); Yi-Tao Yu, PhD (Professor of Biochemistry & Biophysics); Xin Li, PhD (former UR Biochemistry & Biophysics Faculty, currently Professor at Zhejiang University); Sean Lindley (Biology Grad Student, Anderson Lab); Eric Phizicky, PhD (Professor of Biochemistry & Biophysics); Lynne Maquat, PhD (Professor of Biochemistry & Biophysics; Director of the UR CRB ); Eric J. Wagner, PhD (Professor of Biochemistry & Biophysics; Associate Director of the UR CRB); Weifeng Gu, PhD (UR Alumn – PhD 2005, currently Assistant Professor, UCR); Tatsuaki Kurosaki, PhD (Research Assistant Professor, Maquat Lab); Sara Ali (Biophysics Grad Student, Mathews Lab), Yi Pan (Biochemistry Grad Student, Yu Lab)
A large number of UR Center for RNA Biology members and alumni attended the 28th Annual RNA Society Meeting (‘RNA 2023’) held at the Suntec Convention Centre from May 30th-June 4th, 2023. In addition to those pictured, Douglas Turner, PhD, Professor Emeritus of the Department of Biochemistry and Member Emeritus of the Center for RNA Biology, attended and received the 2023 The RNA Society/Cold Spring Harbor Laboratory Press Distinguished Research Mentor Award, which recognizes outstanding mentorship by RNA Society members and highlights the importance of fostering the academic and professional development of trainees in RNA research.
Read More: UR Center for RNA Biology Members and Alumni Attend RNA 2023 in SingaporeSayeeda Zain Spring travel award winners
Tuesday, May 30, 2023
We are pleased to announce the winners of the Sayeeda Zain Spring Travel awards: Brandon Davis, Xueyang He and Matthew Raymonda.



The Sayeeda Zain Travel Award honors the distinguished career and charitable life of Dr. Sayeeda Zain. The award is given in the fall and spring semesters in recognition of research excellence to support travel and related expenses for attendance at a scientific conference or corporate internship to gain practical experience. The next round of Sayeeda Zain Travel Awards will be offered in Fall 2023.
Thank you to all those who applied and congratulations to Brandon, Xueyang and Matt!
A Search Engine for mRNA: Algorithm Identifies Optimal Sequences to Improve COVID Vaccines
Friday, May 19, 2023
Messenger RNA vaccines proved their worth in the COVID pandemic, and new software stands to make the already transformative technology even more powerful.
Scientists developed an algorithm to identify the most stable, efficient mRNA sequences for vaccines. Tests show that the algorithm-derived mRNAs resist deterioration longer, produce more COVID spike protein, and dramatically increase antibody levels in mice compared to currently used mRNA vaccines. The results were reported in the journal Nature.
Study authors believe their tool will be valuable to companies that make mRNA vaccines and to research teams developing mRNA-based therapies for genetic disorders, cancer and a plethora of other diseases that can be treated by using mRNA to express a needed protein.
Searching for the Strongest mRNA
The COVID shots given throughout the pandemic have many advantages—scalable production, safety, efficacy—but suffer from some big drawbacks, including the need for ultra-cold storage and the resultant distribution challenges, and waning immunity. These limitations are due to the fact that mRNAs are inherently unstable and prone to degradation (they are constantly being “eaten” by enzymes present in cells).
The “secret sauce” for creating stronger mRNA sequences requires the right balance of two factors: structure and genetic code. Past research shows that mRNAs with a tight, rigid structure, as opposed to a floppy, unconfined structure, degrade more slowly (structure consolidates mRNAs and provides protection from hungry enzymes). Consequently, they stay in cells for a longer period and have more time to make the desired protein.
The mRNA used in COVID vaccines directs our bodies to make the COVID spike protein. The number of mRNA sequences that encode the spike protein is enormous—larger than the number of atoms in the universe. But, some of these genetic instructions are more efficient than others: one set may allow cells to churn out protein more quickly, while a different set might have redundancies that lead to sluggish protein production.
So, how do you find the right combination of structure and code? RNA expert David Mathews, MD, PhD and computer scientist Liang Huang, PhD, collaborated to create an algorithm that assesses both factors. Like a Google search for mRNA sequences, their algorithm spits out the top result for a specific protein amongst the almost infinite number of possibilities.
“Our tool is designed to identify the best sequence out of a huge space that you could never explore experimentally,” said Mathews, co-corresponding author of the Nature study and the Lynne E. Maquat professor of Biochemistry and Biophysics at the University of Rochester Medical Center. “Prior approaches did a poor job of searching this space. We hope this breakthrough will help companies to develop or improve their mRNA therapies.”
Read More: A Search Engine for mRNA: Algorithm Identifies Optimal Sequences to Improve COVID VaccinesDavid Mathews Publishes Study in Nature on Algorithm to Optimize mRNA Vaccines
Thursday, May 4, 2023
The professor of Biochemistry and Biophysics partnered with an Oregon State University computer scientist to develop the algorithm to help research teams find the most stable, efficient mRNA sequences for vaccines and other therapies, such as monoclonal antibodies and anti-cancer drugs. Tested in COVID-19 mRNA vaccines, the algorithm substantially increased protein expression and antibody levels compared to currently used mRNA sequences.
Read More: David Mathews Publishes Study in Nature on Algorithm to Optimize mRNA VaccinesPulling the Plug on Viral Infections: CRISPR isn’t Just About Cutting
Thursday, April 27, 2023
Study shows how a Cas protein partners with a unique membrane protein to stop viral infection
CRISPR claimed scientific fame for its ability to quickly and accurately edit genes. But, at the core, CRISPR systems are immune systems that help bacteria protect themselves from viruses by targeting and destroying viral DNA and RNA. A new study published in Science reveals a previously unrecognized player in one such system – a membrane protein that enhances anti-viral defense – simultaneously broadening our understanding of and raising more questions related to the complexities of CRISPR.
Uncovering New Clues about CRISPR
CRISPR systems consist of two major components – a guide RNA that targets a specific viral DNA or RNA sequence and a Cas enzyme that cuts the targeted DNA or RNA, preventing a virus from replicating and spreading. A team at the University of Rochester Center for RNA Biology found that a specific Cas protein (Cas13b) not only cuts viral RNA, but communicates with another protein (Csx28) to augment its anti-viral defense.
In partnership with scientists at Cornell, the team discovered that the Csx28 protein forms a pore-like structure (i.e. it has a big hole in it). When they infected E. coli with a phage (virus that attacks bacteria) and deployed the CRISPR-Cas13 system to target and halt infection, they found that Cas13 signals to Csx28 to affect membrane permeability. Once this happens, Csx28 wreaks havoc in the infected cell, discombobulating membrane potential, crushing metabolism and hindering energy production. A virus can’t replicate under such unhospitable circumstances, leading to the team’s conclusion that Csx28 enhances CRISPR-Cas13b’s phage defense.
“This finding upends the idea that CRISPR systems mount their defense only by degrading RNA and DNA in cells and really broadens our view of how CRISPR systems may be working,” said corresponding author Mitchell O’Connell, PhD, assistant professor of Biochemistry and Biophysics at the University of Rochester Medical Center (URMC) and a member of the UR Center for RNA Biology. “When we think about CRISPR, we see Cas proteins such as Cas9 or Cas13 as the big hammer doing all the damage, but that might not be the case; we found that Cas13 and Csx28 are working together to effectively extinguish a virus.”
“When you read this paper you think to yourself…‘what?’ This is such a weird mechanism and not the way I would have predicted that bacteria would work,” added John Lueck, PhD, assistant professor of Pharmacology and Physiology at URMC. “It is really impressive that the team identified this pore-like protein that doesn’t resemble anything else we’ve seen before, and now that we know that this mechanism exists people will start to look for it in other systems. This is exciting because in science, when you scratch the surface, you often find that there is an entirely new world behind it.”
Read More: Pulling the Plug on Viral Infections: CRISPR isn’t Just About CuttingFormer Biochemistry Student Ethan Walker Wins Major Award from the ASBMB
Wednesday, April 5, 2023
 Congratulations to Ethan Walker, who recently was awarded the JBC Herbert Tabor Early Career Investigator Award. Ethan did his Biochemistry Ph.D. work in the laboratory of Sina Ghaemmaghami in the Biology Department at the University of Rochester, and shared his research findings on methionine and protein stability at the 2023 Discover BMB meeting in Seattle Washington. He is currently at Postdoctoral Associate at the Broad Institute if MIT and Harvard, where he is studying drug development for prion diseases. Ethan received his B.S. in Biochemistry at the State University of New York at Oswego, where he graduated Summa Cum Laude.
Congratulations to Ethan Walker, who recently was awarded the JBC Herbert Tabor Early Career Investigator Award. Ethan did his Biochemistry Ph.D. work in the laboratory of Sina Ghaemmaghami in the Biology Department at the University of Rochester, and shared his research findings on methionine and protein stability at the 2023 Discover BMB meeting in Seattle Washington. He is currently at Postdoctoral Associate at the Broad Institute if MIT and Harvard, where he is studying drug development for prion diseases. Ethan received his B.S. in Biochemistry at the State University of New York at Oswego, where he graduated Summa Cum Laude.
Congratulations Ethan!
2023 RNA Institute Symposium: UR Talks and Awards
Tuesday, April 4, 2023
 The 2023 RNA Institute Symposium, a collaborative event hosted by The RNA Institute at the University at Albany (UAlbany) and the University of Rochester (UR) Center for RNA Biology, was held March 16-17, 2023, at UAlbany. This two-day event was co-organized by Lynne Maquat, PhD, Dave Matthews, MD, PhD, Eric Wagner, PhD from the UR Center for RNA Biology, and J. Andrew Berglund, PhD and Marlene Belfort, PhD from The RNA Institute at UAlbany.
The 2023 RNA Institute Symposium, a collaborative event hosted by The RNA Institute at the University at Albany (UAlbany) and the University of Rochester (UR) Center for RNA Biology, was held March 16-17, 2023, at UAlbany. This two-day event was co-organized by Lynne Maquat, PhD, Dave Matthews, MD, PhD, Eric Wagner, PhD from the UR Center for RNA Biology, and J. Andrew Berglund, PhD and Marlene Belfort, PhD from The RNA Institute at UAlbany.
The large-scale event provided a forum for faculty and students to present their research and network with colleagues, featuring speakers from companies with origins in UAlbany and UR research programs, including Codomax, SupreMEtric, Scriptr, and sxRNA.
The Symposium, hosting ~260 attendees, featured 8 trainee talks, 28 lightning talks, 122 posters from students and postdocs/research faculty, and several invited talks by faculty and industry representatives – including Douglas Anderson, PhD of the Aab CVRI and Scriptr Global and Lynne Maquat, PhD, Director of the Center for RNA Biology and Professor in the Department of Biochemistry & Biophysics. Participants from the UR included 38 graduate students, postdocs, and faculty.
As a co-organizer of this event, Dr. Maquat also hosted the Award Ceremony with Thomas Begley, PhD (Associate Director, The RNA Institute at UAlbany). Details on the student talks and the awards given to UR participants are shown below. The overall Symposium schedule and highlights can be found on the event site: https://www.albany.edu/rna/rna-symposium.
UR Participant Photos
Talks
|
UR Student Talks (8 mins + 2 mins Q&A)
|
|
Lily Cisco
Graduate Student, Lueck Lab, CMPP
|
Benefit of Verapamil on Myotonic Dystrophy Bi-Channelopathy
|
|
Xueyang He
Graduate Student, Boutz Lab, BSCB
|
Modeling the Effects of Cancer-Associated Spliceosome Mutations and Identifying Driving Intronic Features using Deep-Learning Neural Networks
|
|
Madeline (Maddie) Jensen
Graduate Student, Wagner Lab, BMB
|
Identification and Structural Basis of a Novel Licensing Factor of the Integrator Cleavage Module
|
|
UR Student and Postdoc Lightning Talks (2 mins + 1 slide)
|
|
Elizabeth Abshire, PhD
Postdoc, Maquat Lab, DBB
|
AKT Constitutes a Signal-Promoted Alternative Exon-Junction Complex that Regulates Nonsense-Mediated mRNA Decay
|
|
Sara Ali
Graduate Student, Mathews Lab, BSCB
|
Mutate2test: New Algorithm and Software for Mutational Design
|
|
Diego Arévalo
Graduate Student, Miller Lab, CHEM
|
Expanding the Known Structure Space for RNA Binding: A Test of 2,5-diketopiperazine
|
|
Dakarai Esgdaille
Graduate Student, Boutz / Mello Labs, BGG
|
Defining the Regulatory Role of Nuclear Paraspeckles in RNA Splicing in the Context of Pancreatic Tumors
|
|
Justin Galardi
Graduate Student, Kielkopf Lab, BMB
|
HIV-1 Rev Regulates Host Transcription and RNA Processing Factor TatSF1 to Promote HIV-1 Infectivity
|
|
Omar Hedaya
Graduate Student, Yao Lab, BMB
|
Secondary Structures that Regulate mRNA Translation Provide Insights for ASO-Mediated Modulation of Protein Expression
|
|
Wooree Ko
Graduate Student, Lueck Lab, CMPP
|
Anticodon-Engineered Transfer RNA for the Treatment of Nonsense-Associated Cystic Fibrosis
|
|
Adrian Molina Vargas
Graduate Student, O’Connell Lab, BGG
|
Development of New Strategies for the Design of Ultrasensitive Cas13a-Based RNA-Diagnostic Tools with Single-Nucleotide Mismatch Sensitivity
|
Awards
|
Only two total Trainee Speaker Awards were given out, one of which was presented to a UR participant:
|
|
Lily Cisco
Graduate Student, Lueck Lab, CMPP
|
Benefit of Verapamil on Myotonic Dystrophy Bi-Channelopathy
|
|
UR Poster Awards (Recipients of cash awards)
|
|
Hironori (Hiro) Adachi, PhD
Staff Scientist/Postdoc, Yu Lab, DBB
|
Targeted Pseudouridylation: An approach for Suppressing Nonsense Mutations in Disease Genes
|
|
Sara Ali
Graduate Student, Mathews Lab, BSCB
|
Mutate2test: New Algorithm and Software for Mutational Design
|
|
Dakarai Esgdaille
Graduate Student, Boutz / Mello Labs, BGG
|
Defining the Regulatory Role of Nuclear Paraspeckles in RNA Splicing in the Context of Pancreatic Tumors
|
|
Jordana Schmierer
Graduate Student, Takimoto Lab, IMV
|
Host Adaptative Mutations in the pH1N1 PA CTD Affect Genome Replication
|
|
Elinore VanGraafeiland
Graduate Student, Miller Lab, BMB
|
Targeting Programmed Ribosomal Frameshifting in SARS-CoV-2 with a Resin-Bound Dynamic Combinatorial Library
|
|
UR RNA Society Awards (recipients of one-year RNA Society Memberships)
|
|
Elizabeth Abshire, PhD
Postdoc, Maquat Lab, DBB
|
AKT Constitutes a Signal-Promoted Alternative Exon-Junction Complex that Regulates Nonsense-Mediated mRNA Decay
|
|
Wooree Ko
Graduate Student, Lueck Lab, CMPP
|
Anticodon-Engineered Transfer RNA for the Treatment of Nonsense-Associated Cystic Fibrosis
|
|
Jessica Perciaccante
Graduate Student, Anderson Lab, BGG
|
The CARDINAL lncRNA Represses TCF-SRF-Mediated Gene Transcription by Co-Occupying SRF-Bound Gene Promoters in the Heart
|
NYS Center for Excellence in RNA Research and Therapeutics (CERRT). This Symposium reflects an ongoing commitment between the University of Rochester Center for RNA Biology and The RNA Institute at the University at Albany-SUNY to train the next generation of RNA Scientists. This endeavor is occurring in real-time and alongside the NYS Biotech industry to develop an academic pipeline to fuel NYS efforts in harnessing RNA as a therapeutic tool and target.
CALL FOR ABSTRACTS: 2023 Young Investigator Symposium on RNA Biology (hosted by Dr. Xin Li)
Friday, March 10, 2023
 Register (free!) and, postdocs/research-track faculty, submit your abstracts by March 20th: 2023
Register (free!) and, postdocs/research-track faculty, submit your abstracts by March 20th: 2023
2023 Young Investigator Symposium on RNA Biology
Dates: March 24-25, 2023
Venue: VIRTUAL via Zoom Webinar. Please register to obtain the link.
Application Deadline: March 20th, 2023
Register and/or Apply Here (All virtual attendees and talk applicants must register.)
The 2023 Young Investigator Symposium on RNA Biology is co-organized by Xin Li, PhD, Associate Professor at the University of Rochester, the RNA Medical Center of Zhejiang University, and the University of Rochester Center for RNA Biology. The Symposium is free to attend and is focused on talks by dedicated postdocs, instructors, and young (associate) researchers from around the world, in addition to the keynote by Yi-Tao Yu, PhD, Professor of Biochemistry & Biophysics at the University of Rochester. The symposium will feature over 20 scholars, each presenting their cutting-edge research for 30 minutes. It will also feature two career sections on networking and job hunting.
Schedule
Friday, March 24th: Virtual format, opening remarks and then talks, starting at 8:20 am EDT
Saturday, March 25th: Virtual talks and Keynote for a half day, starting at 8:30 am EDT
The symposium will also include two virtual career sections on networking and job hunting. Please see the event website for updates on the program and speakers.
Speakers:
(KEYNOTE) Yi-Tao Yu, University of Rochester
Title: Nonsense suppression induced by RNA-guided RNA modification
Qinqin Cui, Zhejiang University
Title: Diverse CMT2 neuropathies are linked to aberrant G3BP interactions in stress granules
Junnan Fang, Emory University
Title: Isoform expression and the post-transcriptional regulations of centrosome Plp mRNA
Wenxia He, University of North Carolina at Chapel Hill
Title: The Degradation Complex on the 3’ End of Histone mRNA
Yanqiang Li, Harvard Medical School
Title: Low RNA stability signifies increased post-transcriptional regulation of cell identity gene
Ling Liu, University of Manitoba, Canada
Title: Mechanism of Adaptive Splicing Controlled by DNA Methylation
Jianjun Luo, Institute of Biophysics, Chinese Academy of Sciences
Title: Systematic Identification and Functional Studies of Long Noncoding RNAs and SEPs
Yicheng Luo, California Institute of Technology
Title: Maternally inherited siRNAs initiate piRNA cluster formation
Maria Mavrikaki, Harvard Medical School, Beth Israel Deaconess Medical Center
Title: Severe COVID-19 is associated with molecular signatures of aging in the human brain
JingRong Zhao, University of California
Title: Molecular profiling of individual FDA-approved clinical drugs identifies modulators of nonsense-mediated mRNA decay
Zhengjie Zhou, The University of Chicago
Title: Vascular targeted mRNA therapies treating cute Respiratory Distress Syndrome
Please send any questions to Xin Li, PhD
Study: Yi-Tao Yu, Paul Boutz Harness Power, Precision of RNA to Make Mutations Invisible
Monday, March 6, 2023
Scientists have discovered a new way to suppress mutations that lead to a wide range of genetic disorders. A study in the journal Molecular Cell describes a strategy that co-opts a normal RNA modification process within cells to transform disease genes into normal genes that produce healthy proteins. The findings are significant because they may ultimately help researchers alter the course of devastating disorders such as cystic fibrosis, muscular dystrophy and many forms of cancer.
Negating Nonsense
Around 15 percent of mutations that lead to genetic diseases are called nonsense mutations. Aptly named, nonsense mutations occur when an mRNA molecule contains an early “stop” signal. When the mRNA takes genetic instructions from DNA to create a protein, this early stop sign orders the cell to stop reading the instructions partway through the process. This results in the creation of an incomplete protein that can lead to disease.

Led by Yi-Tao Yu, PhD, a team of researchers from the University of Rochester Center for RNA Biology designed an artificial guide RNA – a piece of RNA that can modify other types of RNA – to target mRNA molecules that contain early stop signals (also called premature termination codons). Guide RNAs are a natural mechanism that cells use all the time; Yu’s team altered this already existing process.
Like DNA, RNA is made up of molecular building blocks that are represented by the letters A (adenine), G (guanine), U (uracil), and C (cytosine). Premature termination codons always have the building block U in the first position (for example, UAG, UAA or UGA). The team’s artificial guide RNA was designed to modify the U in the first position, changing the molecular makeup of the targeted mRNA so that the stop signal is no longer – or less well – recognized by the cell.
Read More: Study: Yi-Tao Yu, Paul Boutz Harness Power, Precision of RNA to Make Mutations InvisibleRNA Biologist Lynne Maquat Awarded 2023 Gruber Genetics Prize
Thursday, February 23, 2023
Lynne E. Maquat, PhD, the founding director of the Center for RNA Biology at the University of Rochester, has been awarded the 2023 Gruber Genetics Prize for her discovery of nonsense-mediated mRNA decay or NMD in humans. The Gruber International Prize Program, administered by Yale University, honors scientists from around the world whose groundbreaking work leads to fundamental shifts in knowledge and benefits mankind.
Maquat has spent her career deciphering the many roles that RNA plays in sickness and in health, and is best known for elucidating the complexities of NMD in mammalian cells and human disease. One of the major surveillance systems in the body, NMD protects against mistakes in gene expression by targeting and eliminating deleterious mRNAs that could lead to the production of incomplete and potentially toxic proteins. Maquat’s lab also revealed that NMD helps our cells adjust to changes in development and in their environment, and more rapidly respond to certain stimuli.
“Lynne’s scientific prowess and steadfast commitment to her research is exemplary and has helped catapult the field of RNA biology to the forefront of medicine over the past decade,” said Mark B. Taubman, MD, CEO of the University of Rochester Medical Center and dean of the School of Medicine and Dentistry. “This is an exciting time, as Lynne and other scientists are putting her mechanistic findings related to NMD to use to design treatments. She is incredibly deserving of this honor.”
Read More: RNA Biologist Lynne Maquat Awarded 2023 Gruber Genetics PrizeThree Biochemistry and Biophysics Faculty receive Wilmot Cancer Institute 2023 Team Science Pilot Awards
Thursday, February 16, 2023
Congratulations to Department of Biochemistry and Biophysics Professors Josh Munger, Eric Wagner, and Clara Kielkopf, who were each members of separate teams of researchers who received WCI 2023 Science Pilot Awards.
 Josh Munger joins team leader Isaac Harris, Ph.D., and members Calvin Cole, Ph.D., and David Linehan, M.D. in a project entitled “Elucidating the Role of GSH in Cancer-Associated Cachexia”.
Josh Munger joins team leader Isaac Harris, Ph.D., and members Calvin Cole, Ph.D., and David Linehan, M.D. in a project entitled “Elucidating the Role of GSH in Cancer-Associated Cachexia”.
 Eric Wagner joins forces with Brian Marples, Ph.D., professor in the Department of Radiation Oncology, in a project entitled "BRAT1 May Recruit INTS11 RNA Cleavage Activity to Areas of DNA Damage to Impact Local Transcription Complexes”
Eric Wagner joins forces with Brian Marples, Ph.D., professor in the Department of Radiation Oncology, in a project entitled "BRAT1 May Recruit INTS11 RNA Cleavage Activity to Areas of DNA Damage to Impact Local Transcription Complexes”
 Finally, Clara Kielkopf is a member of a large team of researchers lead by Jane Liesveld, M.D., who will focus on a project entitled "The Role of Mesenchymal Stromal Cells and Aging in Myelodysplastic Syndromes”
Finally, Clara Kielkopf is a member of a large team of researchers lead by Jane Liesveld, M.D., who will focus on a project entitled "The Role of Mesenchymal Stromal Cells and Aging in Myelodysplastic Syndromes”
The awards are intended to fund projects that can develop inter-programmatic collaborations, and are aligned with the WCI strategic plan. Each of the projects receives $75,000 for exploratory research.
Work pioneered by Yi-Tao Yu highlighted in new studies
Thursday, February 16, 2023
Rewriting the message: Harnessing RNA pseudouridylation to tackle disease
Matias Montes, Nicole M. Martínez DOI:https://doi.org/10.1016/j.molcel.2023.02.001
Two recent papers in Molecular Cell made strides toward two worthy goals: (1) site-specific modification of RNAs with pseudouridine (Ψ) for functional studies of the epitranscriptome and (2) correction of disease-causing PTCs through Ψ-mediated stop codon readthrough. Both studies build on pioneering work from Yu and colleagues that first showed the potential to engineer small nucleolar RNAs (snoRNAs) for targeted pseudouridylation of stop codons in yeast and discovered that pseudouridylated stop codons suppressed translation termination when artificially introduced in mRNAs.
Read More: Work pioneered by Yi-Tao Yu highlighted in new studiesFormer Graduate of the Biochemistry Ph.D. Program was longtime Professor at Brandeis University
Wednesday, January 11, 2023
 Thomas Clyde Hollocher Jr., a former graduate of the Biochemistry Program at the University of Rochester, passed away on November 3, 2022, at age 91. He received his Ph.D. in 1958, working with Professors Martin Morrison and Elmer Stotz. His thesis was entitled “Kinetic Studies on the Cytochrome C – Cytochrome Oxidase reaction”, where he extended studies of partially soluble systems to reactions in mitochondria capable of oxidative phosphorylation and feedback effects. He went on to a long career at Brandeis University where he taught for 38 years, and lead an active research program in the elucidation and mechanism of bacterial enzymes that carry out denitrification (the reduction of nitrogen oxides to di-nitrogen by bacteria, an alternative to aerobic respiration, and affecting the proportions of nitrogen and oxygen the earth's atmosphere.) He has over 80 research publications, including some related to his interests in chemical paleontology. Most recently Dr. Hollocher held the title of Emeritus Professor of Biochemistry at Brandeis.
Thomas Clyde Hollocher Jr., a former graduate of the Biochemistry Program at the University of Rochester, passed away on November 3, 2022, at age 91. He received his Ph.D. in 1958, working with Professors Martin Morrison and Elmer Stotz. His thesis was entitled “Kinetic Studies on the Cytochrome C – Cytochrome Oxidase reaction”, where he extended studies of partially soluble systems to reactions in mitochondria capable of oxidative phosphorylation and feedback effects. He went on to a long career at Brandeis University where he taught for 38 years, and lead an active research program in the elucidation and mechanism of bacterial enzymes that carry out denitrification (the reduction of nitrogen oxides to di-nitrogen by bacteria, an alternative to aerobic respiration, and affecting the proportions of nitrogen and oxygen the earth's atmosphere.) He has over 80 research publications, including some related to his interests in chemical paleontology. Most recently Dr. Hollocher held the title of Emeritus Professor of Biochemistry at Brandeis.